当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate-controlled C–H or C–C alkynylation of cyclopropanes: generation of aryl radical cations by direct light activation of hypervalent iodine reagents
Chemical Science ( IF 7.6 ) Pub Date : 2022-10-12 , DOI: 10.1039/d2sc04344k Tin V T Nguyen 1 , Matthew D Wodrich 1 , Jerome Waser 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-10-12 , DOI: 10.1039/d2sc04344k Tin V T Nguyen 1 , Matthew D Wodrich 1 , Jerome Waser 1
Affiliation
|
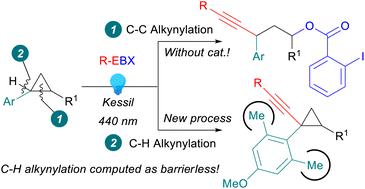
We report the first oxidative C–H alkynylation of arylcyclopropanes. Irradiation of ethynylbenziodoxolone (EBX) reagents with visible light at 440 nm promoted the reaction. By the choice of the aryl group on the cyclopropane, it was possible to completely switch the outcome of the reaction from the alkynylation of the C–H bond to the oxyalkynylation of the C–C bond, which proceeded without the need for a catalyst, in contrast to previous works. The oxyalkynylation could also be extended to aminocyclopropanes as well as styrenes. Computations indicated that the C–H activation became a favoured nearly barrierless process in the presence of two ortho methyl groups on the benzene ring.
中文翻译:

环丙烷的底物控制 C-H 或 C-C 炔基化:通过高价碘试剂的直接光活化产生芳基自由基阳离子
我们报告了芳基环丙烷的第一次氧化 C-H 炔基化。用 440 nm 的可见光照射乙炔基苯并异氧酮 (EBX) 试剂可促进反应。通过选择环丙烷上的芳基,可以将反应结果从 C-H 键的炔基化完全转变为 C-C 键的氧炔基化,无需催化剂即可进行,与以前的作品相反。氧炔基化也可以扩展到氨基环丙烷和苯乙烯。计算表明,在苯环上存在两个邻甲基时,C-H 活化成为一种受欢迎的几乎无障碍的过程。
更新日期:2022-10-12
中文翻译:

环丙烷的底物控制 C-H 或 C-C 炔基化:通过高价碘试剂的直接光活化产生芳基自由基阳离子
我们报告了芳基环丙烷的第一次氧化 C-H 炔基化。用 440 nm 的可见光照射乙炔基苯并异氧酮 (EBX) 试剂可促进反应。通过选择环丙烷上的芳基,可以将反应结果从 C-H 键的炔基化完全转变为 C-C 键的氧炔基化,无需催化剂即可进行,与以前的作品相反。氧炔基化也可以扩展到氨基环丙烷和苯乙烯。计算表明,在苯环上存在两个邻甲基时,C-H 活化成为一种受欢迎的几乎无障碍的过程。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号