当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Bubble-Assisted One-Step Electrodeposition of Cu, Ni, and P toward Electrocatalytic Water Oxidation
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-10-11 , DOI: 10.1021/acsaem.2c02254 Piyush Kumar 1 , Frances Dinsmore 1 , Wujian Miao 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-10-11 , DOI: 10.1021/acsaem.2c02254 Piyush Kumar 1 , Frances Dinsmore 1 , Wujian Miao 1
Affiliation
|
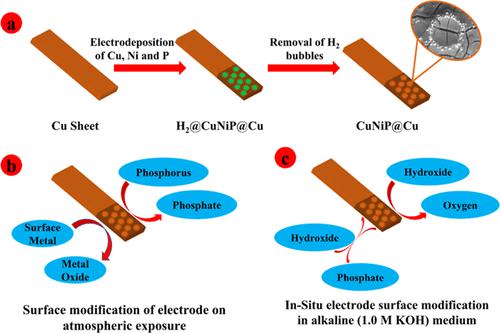
Development of low-cost and highly abundant transition metal-based electrocatalysts for oxygen evolution reaction (OER) activity with low overpotential and high stability is desired for the utilization of electrolytic water-splitting cells to generate hydrogen (H2) fuel. The electrocatalytic activity could be further improved with the fabricated catalyst possessing microporous structures. In this study, binder-free hydrogen bubble-assisted electrodeposition of Cu, Ni, and phosphorous over a Cu sheet (CuNiP@Cu sheet) electrode was constructed. Three electrodeposition solutions consisting of 3:1, 1:1, and 1:3 mole ratios of Cu to Ni and 0.50 M sodium hypophosphite were utilized to produce electrodes under a range of electrodeposition potentials from −2.0 to −9.0 V vs Hg/Hg2SO4 so as to control the rate of hydrogen bubble template generation. The optimum performance for the OER in 1.0 M KOH solution was achieved using the electrode [i.e., CuNiP@Cu (1:1)] generated from an electrodeposition solution consisting of 1:1 mole ratio of Cu to Ni at −4.0 V vs Hg/Hg2SO4 for 5–20 min, which demonstrated a low overpotential value of 318 mV to achieve 10 mA/cm2 current density with a Tafel slope of 100 mV/dec. The potentiodynamic studies of the electrode showed minimal change in the overpotential value for the OER even after 786 cycles at a scan rate of 200 mV/s. The stability was also confirmed with the potentiostatic studies in which the electrode was found to be stable for 20 h of the experimental time. The outcomes suggest that low-cost, readily synthesized, and binder-free hydrogen bubble-assisted one-step electrodeposited microporous electrocatalysts hold excellent features toward the OER.
中文翻译:

氢气气泡辅助一步电沉积 Cu、Ni 和 P 用于电催化水氧化
为了利用电解水分解电池产生氢(H 2)燃料,需要开发具有低过电位和高稳定性的用于析氧反应(OER)活性的低成本和高含量的过渡金属基电催化剂。制备的具有微孔结构的催化剂可以进一步提高电催化活性。在这项研究中,构建了在铜片(CuNiP@Cu 片)电极上的无粘合剂氢气泡辅助电沉积铜、镍和磷。由 3:1、1:1 和 1:3 摩尔比的 Cu 与 Ni 和 0.50 M 次磷酸钠组成的三种电沉积溶液用于在 -2.0 至 -9.0 V vs Hg/Hg 的电沉积电位范围内生产电极2 SO4从而控制氢气泡模板的生成速率。OER 在 1.0 M KOH 溶液中的最佳性能是使用电极 [即 CuNiP@Cu (1:1)] 获得的,该电极由 -4.0 V vs Hg 的 Cu 与 Ni 摩尔比为 1:1 的电沉积溶液组成/Hg 2 SO 4 5-20 分钟,这表明 318 mV 的低过电位值可达到 10 mA/cm 2电流密度,Tafel 斜率为 100 mV/dec。电极的电位动力学研究表明,即使在 200 mV/s 的扫描速率下经过 786 次循环后,OER 的过电位值变化也很小。通过恒电位研究也证实了稳定性,其中电极被发现在实验时间的 20 小时内是稳定的。结果表明,低成本、易于合成和无粘合剂的氢气泡辅助一步电沉积微孔电催化剂具有优异的 OER 特性。
更新日期:2022-10-11
中文翻译:

氢气气泡辅助一步电沉积 Cu、Ni 和 P 用于电催化水氧化
为了利用电解水分解电池产生氢(H 2)燃料,需要开发具有低过电位和高稳定性的用于析氧反应(OER)活性的低成本和高含量的过渡金属基电催化剂。制备的具有微孔结构的催化剂可以进一步提高电催化活性。在这项研究中,构建了在铜片(CuNiP@Cu 片)电极上的无粘合剂氢气泡辅助电沉积铜、镍和磷。由 3:1、1:1 和 1:3 摩尔比的 Cu 与 Ni 和 0.50 M 次磷酸钠组成的三种电沉积溶液用于在 -2.0 至 -9.0 V vs Hg/Hg 的电沉积电位范围内生产电极2 SO4从而控制氢气泡模板的生成速率。OER 在 1.0 M KOH 溶液中的最佳性能是使用电极 [即 CuNiP@Cu (1:1)] 获得的,该电极由 -4.0 V vs Hg 的 Cu 与 Ni 摩尔比为 1:1 的电沉积溶液组成/Hg 2 SO 4 5-20 分钟,这表明 318 mV 的低过电位值可达到 10 mA/cm 2电流密度,Tafel 斜率为 100 mV/dec。电极的电位动力学研究表明,即使在 200 mV/s 的扫描速率下经过 786 次循环后,OER 的过电位值变化也很小。通过恒电位研究也证实了稳定性,其中电极被发现在实验时间的 20 小时内是稳定的。结果表明,低成本、易于合成和无粘合剂的氢气泡辅助一步电沉积微孔电催化剂具有优异的 OER 特性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号