当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Operando Constructing Cu/Cu2O Electrocatalysts for Efficient CO2 Electroreduction to Ethanol: CO2-Assisted Structural Evolution of Octahedral Cu2O by Operando CV Activation
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-11 , DOI: 10.1021/acscatal.2c03833
Yong Yang 1 , Anbang He 1 , Hui Li 1 , Qian Zou 1 , Zuohua Liu 1, 2 , Changyuan Tao 1, 2 , Jun Du 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-11 , DOI: 10.1021/acscatal.2c03833
Yong Yang 1 , Anbang He 1 , Hui Li 1 , Qian Zou 1 , Zuohua Liu 1, 2 , Changyuan Tao 1, 2 , Jun Du 1, 2
Affiliation
|
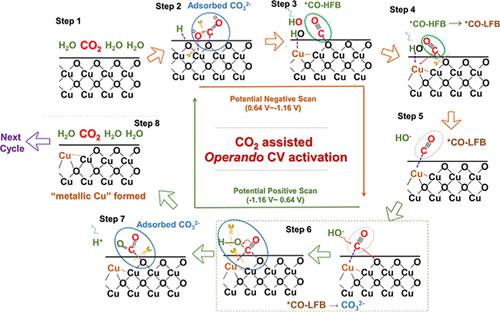
Oxide-derived Cu (OD-Cu) exhibits unique and excellent C2 product selectivity (ethanol, ethylene, etc.) in the field of electrocatalytic reduction reaction of CO2 (eCO2RR), which has a great application value in realizing effective storage of renewable energy and the artificial closed carbon cycle. However, the Cu2O structure encounters complex structure evolution under negative potential conditions, making it difficult to obtain the specific active structures. Here, we found an operando activation strategy for the OD-Cu catalyst based on the cyclic voltammetry (CV) process conducted in CO2-saturated solution. Assisted by an interval eCO2RR, the sluggish octahedral–Cu2O (o-Cu2O) evolves into active Cu/Cu2O–CV, with the formed “metallic Cu” uniformly anchoring on o-Cu2O and accompanied by rich Cuδ+–Cu0 grain boundaries. Combined with in situ and ex situ characterization, compared to o-Cu2O, Cu/Cu2O–CV significantly promoted the formation of C2 products (FEC2 increased from 17.13 to 73.44%). By enhancing the adsorption of CO* and subsequently the formation of O*C*COH intermediates, the Faraday efficiency for ethanol was significantly improved from 5.15% (on o-Cu2O) to 56.56% (on Cu/Cu2O–CV). Our study provides evidence for a previously unexplored function of CO2 in the evolution of o-Cu2O catalysts into highly active structures for the generation of multicarbon products, opening a pathway for the rational design of future catalysts for the electrochemical reduction of CO2.
中文翻译:

Operando 构建 Cu/Cu2O 电催化剂用于高效地将 CO2 电还原为乙醇:通过 Operando CV 活化 CO2 辅助八面体 Cu2O 的结构演化
氧化物衍生Cu(OD-Cu)在CO 2电催化还原反应(eCO 2 RR)领域表现出独特而优异的C 2产物选择性(乙醇、乙烯等),在实现高效的C 2 还原反应方面具有很大的应用价值。可再生能源储存和人工封闭碳循环。然而,Cu 2 O结构在负电位条件下会遇到复杂的结构演化,难以获得特定的活性结构。在这里,我们发现了一种基于在 CO 2饱和溶液中进行的循环伏安法 (CV) 过程的 OD-Cu 催化剂的操作活化策略。由间隔 eCO 2辅助RR,缓慢的八面体-Cu 2 O ( o -Cu 2 O) 演化为活性Cu/Cu 2 O-CV,形成的“金属Cu”均匀锚定在o -Cu 2 O上,并伴有丰富的Cu δ+ - Cu 0晶界。结合原位和异位表征,与o - Cu 2 O相比,Cu/Cu 2 O-CV显着促进了C 2产物(FE C2从 17.13% 增加到 73.44%)。通过增强 CO* 的吸附并随后形成 O*C*COH 中间体,乙醇的法拉第效率从 5.15%(在o - Cu 2 O 上)显着提高到 56.56%(在 Cu/Cu 2 O-CV上) )。我们的研究为 CO 2在o -Cu 2 O 催化剂演变为高活性结构以生成多碳产物中的未探索功能提供了证据,为未来用于电化学还原 CO 2的催化剂的合理设计开辟了一条途径.
更新日期:2022-10-11
中文翻译:

Operando 构建 Cu/Cu2O 电催化剂用于高效地将 CO2 电还原为乙醇:通过 Operando CV 活化 CO2 辅助八面体 Cu2O 的结构演化
氧化物衍生Cu(OD-Cu)在CO 2电催化还原反应(eCO 2 RR)领域表现出独特而优异的C 2产物选择性(乙醇、乙烯等),在实现高效的C 2 还原反应方面具有很大的应用价值。可再生能源储存和人工封闭碳循环。然而,Cu 2 O结构在负电位条件下会遇到复杂的结构演化,难以获得特定的活性结构。在这里,我们发现了一种基于在 CO 2饱和溶液中进行的循环伏安法 (CV) 过程的 OD-Cu 催化剂的操作活化策略。由间隔 eCO 2辅助RR,缓慢的八面体-Cu 2 O ( o -Cu 2 O) 演化为活性Cu/Cu 2 O-CV,形成的“金属Cu”均匀锚定在o -Cu 2 O上,并伴有丰富的Cu δ+ - Cu 0晶界。结合原位和异位表征,与o - Cu 2 O相比,Cu/Cu 2 O-CV显着促进了C 2产物(FE C2从 17.13% 增加到 73.44%)。通过增强 CO* 的吸附并随后形成 O*C*COH 中间体,乙醇的法拉第效率从 5.15%(在o - Cu 2 O 上)显着提高到 56.56%(在 Cu/Cu 2 O-CV上) )。我们的研究为 CO 2在o -Cu 2 O 催化剂演变为高活性结构以生成多碳产物中的未探索功能提供了证据,为未来用于电化学还原 CO 2的催化剂的合理设计开辟了一条途径.

































 京公网安备 11010802027423号
京公网安备 11010802027423号