Chemosphere ( IF 8.1 ) Pub Date : 2022-10-11 , DOI: 10.1016/j.chemosphere.2022.136820 Zhengdi Wu 1 , Fei Lou 2 , Yubin Tang 3 , Huiyu Dong 2 , Zhimin Qiang 2
|
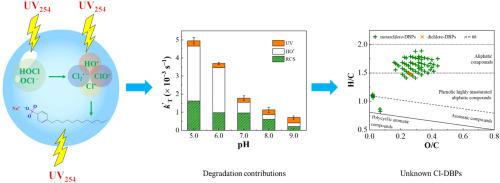
The degradation kinetics of Sodium dodecylbenzene sulfonate (SDBS) surfactant in the UV/chlorine process was comprehensively investigated, and the formation of chlorinated disinfection by-products (Cl-DBPs) were determined. Results showed that the degradation of SDBS by UV, chlorine and UV/chlorine all followed pseudo-first-order kinetics. The rate constant by UV/chlorine in ultrapure water was approximately 3 times higher than the sum of those by UV and chlorine, and decreased from 0.297 to 0.063 min−1 with pH increasing from 5.0 to 9.0. Water matrices such as NO3−, HCO3− and natural organic matter (NOM) inhibited the degradation efficiency to a certain extent. The second-order rate constant of SDBS with HO• was determined as 2.84 × 109 M−1 s−1. Through using different probes, the main contributors to SDBS degradation were found to be UV, HO• and reactive chlorine species (RCS). Meanwhile, 64.0 μg L−1 trichloromethane (TCM) and 8.7 μg L−1 chloral hydrate (CH) were simultaneously formed within 30 min of UV/chlorine treatment. The concentration of total organic chlorine (TOCl) (424.0 μg L−1) was obviously higher than those of TCM and CH. In addition, 414 unknown by-products formed during UV/chlorine treatment were detected by mass spectrometry at a high confidence level, including 64 monochloro-DBPs and 2 dichloro-DBPs. Although UV/chlorine process accelerated SDBS degradation, the associated DBP formation deserves enough attention.
中文翻译:

紫外/氯工艺中十二烷基苯磺酸钠表面活性剂的加速转化:动力学和氯化消毒副产物的形成
全面研究了十二烷基苯磺酸钠(SDBS)表面活性剂在紫外/氯过程中的降解动力学,并确定了氯化消毒副产物(Cl-DBPs)的形成。结果表明,紫外、氯和紫外/氯对SDBS的降解均遵循准一级动力学。紫外/氯在超纯水中的速率常数大约是紫外和氯之和的 3 倍,随着 pH 从 5.0 增加到 9.0 ,速率常数从 0.297 下降到 0.063 min -1 。NO 3 -、HCO 3 -和天然有机物(NOM)等水基质在一定程度上抑制了降解效率。SDBS 与 H2O 的二阶速率常数•确定为2.84 × 10 9 M -1 s -1。通过使用不同的探针,发现SDBS 降解的主要贡献者是UV、H2O •和活性氯物种(RCS)。同时,在紫外线/氯处理的 30 分钟内同时形成 64.0 μg L -1三氯甲烷(TCM)和 8.7 μg L -1水合氯醛(CH)。总有机氯(TOCl)浓度(424.0 μg L -1) 明显高于 TCM 和 CH。此外,通过质谱法以高置信水平检测到在紫外/氯处理过程中形成的 414 种未知副产物,包括 64 种一氯-DBP 和 2 种二氯-DBP。尽管紫外/氯过程加速了 SDBS 的降解,但相关的 DBP 形成值得关注。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号