Coordination Chemistry Reviews ( IF 20.3 ) Pub Date : 2022-10-11 , DOI: 10.1016/j.ccr.2022.214827 Vishakha Goyal , Naina Sarki , Anand Narani , Ganesh Naik , Kishore Natte , Rajenahally V. Jagadeesh
|
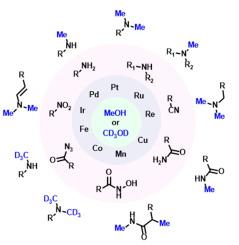
N-methylamine functionalities are valuable motifs, which play vital roles in the properties and activities of essential fine and bulk chemicals including molecules used in life science applications, and functional materials. Thus, N-methylations constitute an important class of reactions in organic synthesis and drug discovery, giving access to advanced compounds, pharmaceuticals, biomolecules, and agrochemicals. The diversity of these kinds of amine structures and their biological relevance stimulated researchers in academia and industry to develop more sustainable, atom-economical, and cost-effective methodologies for the synthesis of N-methylated molecules and pharmaceutical agents. For their preparation, N-methylation using methanol represents a convenient and resourceful methodology because methanol is an abundantly available bulk chemical and serves as an effectual methyl (–CH3) source. Moreover, methanol is less hazardous and produces water as the only by-product in methylation reactions. In this regard, in recent years, several discoveries have been made on the catalytic valorization of methanol as a powerful methylation reagent. This review aims to provide the most recent progress made in catalytic N-methylation of nitrogen-containing molecules employing methanol as a key C1 source from 2017 to August 2022. In particular, the synthesis of N-methylamines and related bioactive compounds starting from different organo-nitrogen compounds such as amines, nitroarenes, amides, sulfonamides, aldoximes, nitriles, and acyl azides using both homogeneous and heterogeneous catalysts including photo(redox) systems are discussed in detail. In addition, N-trideuteromethylation of amines or nitroarenes using deuterated methanol is described as a versatile synthetic tool for synthesizing N-trideuteromethyl labelled molecules, which play significant roles in pharmacological and metabolic activities. We sincerely hope that this review will be interesting and beneficial to scientists working in both academic research and industries in the areas of organic synthesis, medicinal, and biological chemistry.
中文翻译:

使用甲醇和氘代甲醇催化 N-甲基化和 N-三氘甲基化反应的最新进展
N-甲胺官能团是有价值的基序,它们在基本精细和大宗化学品(包括用于生命科学应用的分子和功能材料)的性质和活性中发挥着至关重要的作用。因此,N-甲基化在有机合成和药物发现中构成了一类重要的反应,为获得先进的化合物、药物、生物分子和农用化学品提供了途径。这些胺结构的多样性及其生物学相关性促使学术界和工业界的研究人员开发出更可持续、原子经济和成本效益更高的方法来合成N-甲基化分子和药剂。为了他们的准备,N 使用甲醇进行甲基化是一种方便且资源丰富的方法,因为甲醇是一种可广泛使用的大宗化学品,可作为有效的甲基 (-CH 3 ) 来源。此外,甲醇的危害较小,并且在甲基化反应中产生水作为唯一的副产物。在这方面,近年来,在甲醇作为强甲基化试剂的催化增值方面取得了一些发现。本综述旨在提供2017 年至 2022 年 8 月期间以甲醇为关键 C1 源的含氮分子催化N 甲基化的最新进展。特别是N的合成 详细讨论了使用均相和多相催化剂(包括光(氧化还原)系统)从不同的有机氮化合物(例如胺、硝基芳烃、酰胺、磺酰胺、醛肟、腈和酰基叠氮化物)开始的甲胺和相关生物活性化合物。此外,使用氘代甲醇对胺或硝基芳烃进行N-三氘甲基化被描述为用于合成N-三氘甲基标记分子的通用合成工具,这些分子在药理和代谢活性中起重要作用。我们真诚地希望这篇综述对从事有机合成、药物和生物化学领域的学术研究和行业的科学家们来说是有趣和有益的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号