Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-standing Fe3O4 decorated paper electrode as a binder-free trifunctional electrode for electrochemical ammonia synthesis and Zn–O2 batteries
Nanoscale ( IF 5.8 ) Pub Date : 2022-10-11 , DOI: 10.1039/d2nr03297j Alankar Kafle 1 , Divyani Gupta 1 , Ankur Bordoloi 2 , Tharamani C Nagaiah 1
Nanoscale ( IF 5.8 ) Pub Date : 2022-10-11 , DOI: 10.1039/d2nr03297j Alankar Kafle 1 , Divyani Gupta 1 , Ankur Bordoloi 2 , Tharamani C Nagaiah 1
Affiliation
|
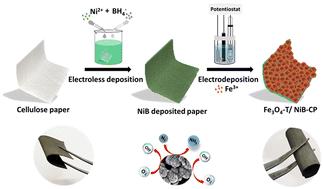
The conversion of the abundant biodegradable material into electroactive electrode material can be a good resource for sustainable energy conversion and storage applications. Herein, we present a simple, cost-effective and green approach for the fabrication of a flexible cellulose paper electrode using an electroless-electrodeposition method. The one-step electroless deposition route is followed to induce conductivity into a non-conductive cellulose paper substrate without using any expensive activators or sensitisers. The Fe3O4 is then electro-deposited as an active catalyst over the conductive paper substrate for use in electrochemical activities. The as-fabricated paper electrode shows promising activity and stability during the dinitrogen reduction reaction (NRR) as well as oxygen bifunctional electrocatalysis. A faradaic efficiency of 4.32% with a yield rate of 245 μg h−1 mgcat−1 at −0.1 V is achieved for NRR whereas a very small overpotential of 180 mV is required to reach 10 mA cm−2 during OER, and the ORR reaction starts at the onset potential of 0.86 V. The practical applicability of the paper electrode is validated by assembling a Zn–O2 battery showing a peak power density of 81 mW cm−2 and a stability up to 35 h during charge–discharge cycles, which can power the NRR to produce NH3 under full cell conditions.
中文翻译:

自支撑 Fe3O4 装饰纸电极作为电化学氨合成和 Zn-O2 电池的无粘合剂三功能电极
将丰富的可生物降解材料转化为电活性电极材料可以成为可持续能源转换和存储应用的良好资源。在此,我们提出了一种使用化学电沉积方法制造柔性纤维素纸电极的简单、经济高效且绿色的方法。遵循一步化学沉积路线,在不使用任何昂贵的活化剂或敏化剂的情况下,将导电性引入非导电纤维素纸基材。Fe 3 O 4然后将其作为活性催化剂电沉积在导电纸基材上,用于电化学活动。制造的纸电极在二氮还原反应 (NRR) 以及氧双功能电催化过程中显示出良好的活性和稳定性。NRR 在 -0.1 V 时实现了 4.32% 的法拉第效率和 245 μg h -1 mg cat -1的产率,而在 OER 期间达到 10 mA cm -2需要非常小的 180 mV 过电势,并且ORR 反应在 0.86 V 的起始电位开始。纸电极的实际适用性通过组装 Zn-O 2电池得到验证,峰值功率密度为 81 mW cm -2并且在充放电循环期间的稳定性长达 35 小时,这可以为 NRR 提供动力以在全电池条件下产生 NH 3 。
更新日期:2022-10-11
中文翻译:

自支撑 Fe3O4 装饰纸电极作为电化学氨合成和 Zn-O2 电池的无粘合剂三功能电极
将丰富的可生物降解材料转化为电活性电极材料可以成为可持续能源转换和存储应用的良好资源。在此,我们提出了一种使用化学电沉积方法制造柔性纤维素纸电极的简单、经济高效且绿色的方法。遵循一步化学沉积路线,在不使用任何昂贵的活化剂或敏化剂的情况下,将导电性引入非导电纤维素纸基材。Fe 3 O 4然后将其作为活性催化剂电沉积在导电纸基材上,用于电化学活动。制造的纸电极在二氮还原反应 (NRR) 以及氧双功能电催化过程中显示出良好的活性和稳定性。NRR 在 -0.1 V 时实现了 4.32% 的法拉第效率和 245 μg h -1 mg cat -1的产率,而在 OER 期间达到 10 mA cm -2需要非常小的 180 mV 过电势,并且ORR 反应在 0.86 V 的起始电位开始。纸电极的实际适用性通过组装 Zn-O 2电池得到验证,峰值功率密度为 81 mW cm -2并且在充放电循环期间的稳定性长达 35 小时,这可以为 NRR 提供动力以在全电池条件下产生 NH 3 。































 京公网安备 11010802027423号
京公网安备 11010802027423号