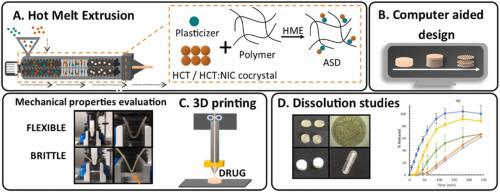
材料挤出 (ME) 技术在制药领域的应用面临的最大挑战之一是缺乏具有药物质量的即用型聚合材料(长丝)。为了克服这一挑战,材料挤出可以与热熔挤出 (HME) 工艺相结合,从而能够使用药物批准的聚合物和掺入药物来生产长丝。这份手稿介绍了含高熔点 API(氢氯噻嗪、HCT、T m= 266–268 °C)与两种药学上批准的聚合物(聚乙烯醇、PVA 和醋酸琥珀酸羟丙基甲基纤维素、HPMCAS)通过组合 HME 和 ME 处理具有不同的技术和药学特性。通过获得具有较低熔点 (T m= 173.3 °C) 比起始材料高。使用了两种增塑剂——用于 HPMCAS 的柠檬酸三乙酯 (TEC) 和用于 PVA 的山梨糖醇 (SOR)。使用融合方法制备含有 10–50 wt% 增塑剂的共混物,并使用 PXRD、DSC 和 FTIR 进行彻底分析,从而能够选择用于进一步 HME 加工的配方。获得了具有 10-30 wt% 增塑剂的安慰剂长丝,并评估了它们的机械性能和 ME 可加工性,以选择具有所需工艺参数的聚合物共混物。最后,生产含有 20 wt% 增塑剂与 HCT 或 HCT:NIC 共晶的长丝,并使用 ME 加工成片剂。使用 PXRD、DSC、和 FTIR,然后用偏光显微镜和 SEM 进行材料成像。共晶形成不仅能够改变药物的熔点以匹配两个过程的温度,而且还改善了细丝的机械性能,这对于 ME 加工很重要。在基于 PVA 的配方的情况下,共晶体在 HME 加工过程中变成无定形,形成柔性和可印刷的细丝。相比之下,嵌入基于 HPMCAS 的细丝中的药物形成了影响挤出物机械性能的晶体。与使用常规方法(即压片和包封)获得的材料相比,获得的片剂的机械性能和药物从 AM 片剂中的释放曲线得到了增强。共晶形成不仅能够改变药物的熔点以匹配两个过程的温度,而且还改善了细丝的机械性能,这对于 ME 加工很重要。在基于 PVA 的配方的情况下,共晶体在 HME 加工过程中变成无定形,形成柔性和可印刷的细丝。相比之下,嵌入基于 HPMCAS 的细丝中的药物形成了影响挤出物机械性能的晶体。与使用常规方法(即压片和包封)获得的材料相比,获得的片剂的机械性能和药物从 AM 片剂中的释放曲线得到了增强。共晶形成不仅能够改变药物的熔点以匹配两个过程的温度,而且还改善了细丝的机械性能,这对于 ME 加工很重要。在基于 PVA 的配方的情况下,共晶体在 HME 加工过程中变成无定形,形成柔性和可印刷的细丝。相比之下,嵌入基于 HPMCAS 的细丝中的药物形成了影响挤出物机械性能的晶体。与使用常规方法(即压片和包封)获得的材料相比,获得的片剂的机械性能和药物从 AM 片剂中的释放曲线得到了增强。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Adjusting the melting point of an Active Pharmaceutical Ingredient (API) via cocrystal formation enables processing of high melting drugs via combined hot melt and materials extrusion (HME and ME)
One of the biggest challenges for the application of materials extrusion (ME) technology in the pharmaceutical sector is the lack of ready-to-use polymeric materials (filaments) of pharmaceutical quality. To overcome this challenge materials extrusion can be combined with the Hot Melt Extrusion (HME) process enabling the production of filaments using pharmaceutically approved polymers with incorporated drugs. This manuscript presents a step by step approach for the formulation of additively manufactured tablets containing a high melting point API (hydrochlorothiazide, HCT, Tm = 266–268 °C) with two pharmaceutically approved polymers (polyvinyl alcohol, PVA and hydroxypropyl methylcellulose acetate succinate, HPMCAS) of different technological and pharmaceutical properties via combined HME and ME processing. The thermal properties of a model drug were adapted to the processing window of both HME and ME by obtaining a hydrochlorothiazide: nicotinamide cocrystal (HCT:NIC) with a lower melting point (Tm = 173.3 °C) than the starting material. Two plasticizers were used - triethyl citrate (TEC) for HPMCAS and sorbitol (SOR) for PVA. Blends containing 10–50 wt% of plasticizer were prepared using the fusion method and thoroughly analysed using PXRD, DSC, and FTIR enabling the selection of formulations for further HME processing. Placebo filaments with 10–30 wt% of plasticizer were obtained and their mechanical properties and ME processability were assessed to select a polymer blend with desirable process parameters. Finally, the filaments containing 20 wt% of plasticizer with HCT or the HCT:NIC cocrystal were produced and processed using ME to form tablets. The phase of the drug (crystalline or amorphous), mechanical properties of filaments and tablets as well as the drug content in the obtained materials were assessed using PXRD, DSC, and FTIR followed by materials imaging with polarising microscopy and SEM. The cocrystal formation not only enabled to modify the melting point of the drug to match the temperature of both processes but also improved the mechanical properties of the filaments which is important for ME processing. In the case of PVA based formulations the cocrystal turned amorphous upon HME processing forming flexible and printable filaments. In contrast, the drug embedded in HPMCAS based filaments formed crystals that affected the mechanical properties of the extrudates. The mechanical properties of the obtained tablets and the release profile of the drug from the AM tablets were enhanced as compared to the materials obtained using conventional methods, i.e. tableting and encapsulation.








































 京公网安备 11010802027423号
京公网安备 11010802027423号