当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Tetrahydro-2H-thiopyran 1,1-Dioxides via [1+1+1+1+1+1] Annulation: An Unconventional Usage of a Tethered C–S Synthon
Organic Letters ( IF 4.9 ) Pub Date : 2022-10-10 , DOI: 10.1021/acs.orglett.2c03194 Xiang-Long Chen 1 , Huai-Yu Wang 1 , Chun-Yan Wu 1 , Bo-Cheng Tang 2 , Yao-Luo Hu 1 , Jin-Tian Ma 1 , Shi-Yi Zhuang 1 , Zhi-Cheng Yu 1 , Yan-Dong Wu 1 , An-Xin Wu 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-10-10 , DOI: 10.1021/acs.orglett.2c03194 Xiang-Long Chen 1 , Huai-Yu Wang 1 , Chun-Yan Wu 1 , Bo-Cheng Tang 2 , Yao-Luo Hu 1 , Jin-Tian Ma 1 , Shi-Yi Zhuang 1 , Zhi-Cheng Yu 1 , Yan-Dong Wu 1 , An-Xin Wu 1
Affiliation
|
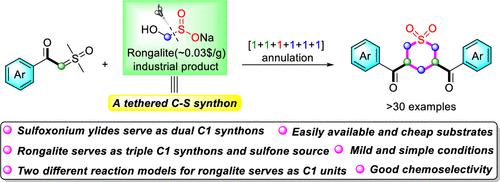
An unprecedented [1+1+1+1+1+1] annulation process has been developed for the construction of tetrahydro-2H-thiopyran 1,1-dioxides. Notably, rongalite acted as a tethered C–S synthon in this reaction and can be chemoselectively used as triple C1 units and as a source of sulfone. Mechanistic investigation indicated that two different carbon-increasing models are involved in this reaction in which rongalite serves as C1 units.
中文翻译:

通过 [1+1+1+1+1+1] 环化合成 Tetrahydro-2H-thiopyran 1,1-Dioxides:束缚 C-S 合成子的非常规用途
已经开发出一种前所未有的 [1+1+1+1+1+1] 环化工艺来构建四氢-2 H-噻喃 1,1-二氧化物。值得注意的是,雕白粉在该反应中充当束缚的 C-S 合成子,可以化学选择性地用作三重 C1 单元和砜的来源。机理研究表明,该反应涉及两种不同的增碳模型,其中雕白粉作为C1单元。
更新日期:2022-10-10
中文翻译:

通过 [1+1+1+1+1+1] 环化合成 Tetrahydro-2H-thiopyran 1,1-Dioxides:束缚 C-S 合成子的非常规用途
已经开发出一种前所未有的 [1+1+1+1+1+1] 环化工艺来构建四氢-2 H-噻喃 1,1-二氧化物。值得注意的是,雕白粉在该反应中充当束缚的 C-S 合成子,可以化学选择性地用作三重 C1 单元和砜的来源。机理研究表明,该反应涉及两种不同的增碳模型,其中雕白粉作为C1单元。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号