当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Practical Polymer Electrolyte for Lithium and Sodium Batteries: Poly(pentyl malonate)
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-10-10 , DOI: 10.1021/acsenergylett.2c02128 Xiaopeng Yu 1, 2 , Zach J. Hoffman 1, 2 , Jaeyong Lee 1, 2 , Chao Fang 1, 2 , Lily A. Gido 1, 2 , Vivaan Patel 1, 2 , Hany B. Eitouni 3 , Rui Wang 1, 2 , Nitash P. Balsara 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2022-10-10 , DOI: 10.1021/acsenergylett.2c02128 Xiaopeng Yu 1, 2 , Zach J. Hoffman 1, 2 , Jaeyong Lee 1, 2 , Chao Fang 1, 2 , Lily A. Gido 1, 2 , Vivaan Patel 1, 2 , Hany B. Eitouni 3 , Rui Wang 1, 2 , Nitash P. Balsara 1, 2
Affiliation
|
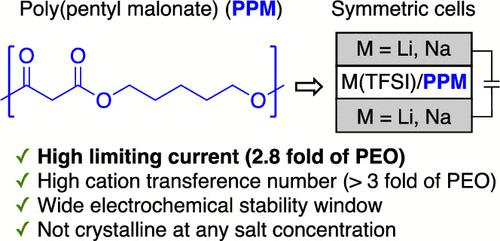
The rate at which rechargeable batteries can be charged and discharged depends primarily on ion transport between the electrodes. This is governed by the limiting current and electrochemical stability of the electrolyte. To our knowledge, the limiting current values of all dry polymer electrolytes in the literature are either not reported or lower than that of the benchmark polymer electrolyte comprising poly(ethylene oxide) (PEO) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). We describe the synthesis and characterization of a new polymer electrolyte, poly(pentyl malonate) (PPM). The length-normalized limiting current of PPM/LiTFSI in lithium-polymer-lithium symmetric cells is about a factor of 2.8 higher than that of PEO/LiTFSI at the same salt concentration and temperature. This polymer also exhibits increased electrochemical stability at high potentials (up to 5 V against Li+/Li) relative to PEO. Promising preliminary data obtained in sodium-polymer-sodium symmetric cells is also presented.
中文翻译:

一种实用的锂电池和钠电池聚合物电解质:聚丙二酸戊酯
可充电电池的充电和放电速率主要取决于电极之间的离子传输。这取决于电解质的极限电流和电化学稳定性。据我们所知,文献中所有干聚合物电解质的极限电流值要么没有报道,要么低于包含聚(环氧乙烷)(PEO)和双(三氟甲磺酰)亚胺锂(LiTFSI)的基准聚合物电解质的极限电流值。我们描述了一种新的聚合物电解质聚(丙二酸戊酯)(PPM)的合成和表征。在相同盐浓度和温度下,PPM/LiTFSI 在锂-聚合物-锂对称电池中的长度归一化限制电流比 PEO/LiTFSI 高约 2.8 倍。+ /Li) 相对于 PEO。还提供了在钠-聚合物-钠对称细胞中获得的有希望的初步数据。
更新日期:2022-10-10
中文翻译:

一种实用的锂电池和钠电池聚合物电解质:聚丙二酸戊酯
可充电电池的充电和放电速率主要取决于电极之间的离子传输。这取决于电解质的极限电流和电化学稳定性。据我们所知,文献中所有干聚合物电解质的极限电流值要么没有报道,要么低于包含聚(环氧乙烷)(PEO)和双(三氟甲磺酰)亚胺锂(LiTFSI)的基准聚合物电解质的极限电流值。我们描述了一种新的聚合物电解质聚(丙二酸戊酯)(PPM)的合成和表征。在相同盐浓度和温度下,PPM/LiTFSI 在锂-聚合物-锂对称电池中的长度归一化限制电流比 PEO/LiTFSI 高约 2.8 倍。+ /Li) 相对于 PEO。还提供了在钠-聚合物-钠对称细胞中获得的有希望的初步数据。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号