International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2022-10-03 , DOI: 10.1016/j.ijbiomac.2022.09.283 Shuwen Xu 1 , Zi'an Feng 1 , Yue Zhang 1 , Haiyu Ni 1 , Zhenguang Liu 1 , Deyun Wang 1
|
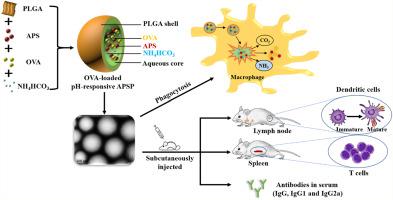
现代医学认为,黄芪多糖(APS)具有增强免疫力的功效,但其特殊性大大降低了临床应用。聚(乳酸--glycolic acid) (PLGA) 是一种合成载体材料,具有突出的生化特性。在本研究中,PLGA 材料被用于制备新型的 pH 响应靶向药物递送载体,其内部封装了 APS。OVA-loaded pH-responsive APS-encapsulated PLGA Nanoparticles (OVA-loaded pH-responsive APSPs) 和 OVA-loaded APSPs 采用多重乳液溶剂挥发法构建。在体外和体内评估了 PLGA 纳米颗粒 (NP) 的表征和免疫增强活性。NP 的大小范围为 142.6 至 194.6 nm,并且所有 NP 都带负电荷。此外,pH 响应性 APSP 在酸性环境中表现出剧烈的释放行为。pH 响应性 APSP 具有低细胞毒性,并显着增强 MHC-II、CD80、CD86、和巨噬细胞的吞噬能力。与单独使用 APS 相比,两种负载 OVA 的 NPs 都可以刺激更强的 Th1 偏向免疫反应,并且它们可以分别显着促进小鼠脾淋巴细胞和树突状细胞的增殖、分化和成熟。NPs 诱导显着增强的抗原特异性 IgG 抗体反应和 IL-4、IL-6、IFN-γ 和 TNF-α 的表达。此外,加载 OVA 的 pH 响应 APSP 在早期免疫期间具有增强细胞和体液免疫的能力,而加载 OVA 的 APSP 在免疫反应的后期阶段具有优势。小鼠脾淋巴细胞和树突状细胞的成熟度。NPs 诱导显着增强的抗原特异性 IgG 抗体反应和 IL-4、IL-6、IFN-γ 和 TNF-α 的表达。此外,加载 OVA 的 pH 响应 APSP 在早期免疫期间具有增强细胞和体液免疫的能力,而加载 OVA 的 APSP 在免疫反应的后期阶段具有优势。小鼠脾淋巴细胞和树突状细胞的成熟度。NPs 诱导显着增强的抗原特异性 IgG 抗体反应和 IL-4、IL-6、IFN-γ 和 TNF-α 的表达。此外,加载 OVA 的 pH 响应 APSP 在早期免疫期间具有增强细胞和体液免疫的能力,而加载 OVA 的 APSP 在免疫反应的后期阶段具有优势。

"点击查看英文标题和摘要"


















































 京公网安备 11010802027423号
京公网安备 11010802027423号