当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics and Mechanism of Lithium Extraction from α-Spodumene in Potassium Hydroxide Solution
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-10-06 , DOI: 10.1021/acs.iecr.2c02019 Shengbo Qiu 1 , Yue Zhu 1 , Youfa Jiang 1 , Chenglin Liu 1, 2 , Jianguo Yu 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-10-06 , DOI: 10.1021/acs.iecr.2c02019 Shengbo Qiu 1 , Yue Zhu 1 , Youfa Jiang 1 , Chenglin Liu 1, 2 , Jianguo Yu 1, 2
Affiliation
|
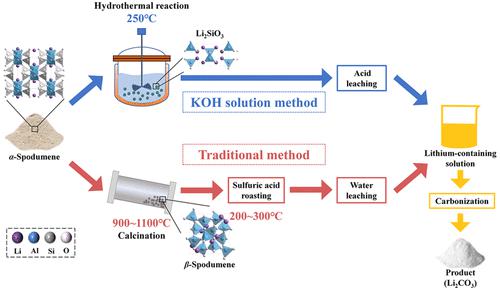
Lithium extraction from α-spodumene in a potassium hydroxide solution was proposed to provide a new green metallurgical process for spodumene concentrate. The structure of α-spodumene could be destroyed directly by a KOH solution, and new solid-phase products of Li2SiO3 and KAlSiO4 were generated simultaneously. The total lithium extraction efficiency could reach 89.9%, of which 84.1% was converted into Li2SiO3 and 5.8% converted into the liquid phase under optimal conditions: initial KOH concentration of 50 wt %, stirring speed of 500 rpm, mass ratio of KOH/ore of 2:1, leaching temperature of 523.15 K, and leaching time of 16 h. The experimental data fitted well with the Avrami–Erofeev equation model, –ln(1 – X) = (Kt)n. The apparent activation energy and Avrami index were calculated as 106.37 kJ·mol–1 and 0.80, respectively.
中文翻译:

氢氧化钾溶液中α-锂辉石提锂的动力学和机理
提出了在氢氧化钾溶液中从α-锂辉石中提取锂,为锂辉石精矿提供了一种新的绿色冶金工艺。KOH溶液可以直接破坏α-锂辉石的结构,同时生成新的固相产物Li 2 SiO 3和KAlSiO 4。总提锂效率可达89.9%,其中84.1%转化为Li 2 SiO 3,5.8%转化为液相最佳条件:初始KOH浓度为50 wt %,搅拌速度为500 rpm,质量比为KOH/矿石2:1,浸出温度523.15 K,浸出时间16 h。实验数据与 Avrami-Erofeev 方程模型拟合良好,– ln(1 – X ) = ( Kt ) n。表观活化能和 Avrami 指数分别计算为 106.37 kJ·mol –1和 0.80。
更新日期:2022-10-06
中文翻译:

氢氧化钾溶液中α-锂辉石提锂的动力学和机理
提出了在氢氧化钾溶液中从α-锂辉石中提取锂,为锂辉石精矿提供了一种新的绿色冶金工艺。KOH溶液可以直接破坏α-锂辉石的结构,同时生成新的固相产物Li 2 SiO 3和KAlSiO 4。总提锂效率可达89.9%,其中84.1%转化为Li 2 SiO 3,5.8%转化为液相最佳条件:初始KOH浓度为50 wt %,搅拌速度为500 rpm,质量比为KOH/矿石2:1,浸出温度523.15 K,浸出时间16 h。实验数据与 Avrami-Erofeev 方程模型拟合良好,– ln(1 – X ) = ( Kt ) n。表观活化能和 Avrami 指数分别计算为 106.37 kJ·mol –1和 0.80。






























 京公网安备 11010802027423号
京公网安备 11010802027423号