当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 5,7-Dihydro-6H-pyrrolo[2,3-d]pyrimidin-6-ones as Highly Selective CDK2 Inhibitors
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2022-10-06 , DOI: 10.1021/acsmedchemlett.2c00408 Alexander Sokolsky 1 , Sarah Winterton 1 , Keith Kennedy 1 , Katherine Drake 1 , Kristine Stump 1 , Lu Huo 1 , Yvonne Lo 1 , Min Ye 1 , Maryanne Covington 1 , Sharon Diamond 1 , Yan-Ou Yang 1 , Sunkyu Kim 1 , Swamy Yeleswaram 1 , Liangxing Wu 1 , Wenqing Yao 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2022-10-06 , DOI: 10.1021/acsmedchemlett.2c00408 Alexander Sokolsky 1 , Sarah Winterton 1 , Keith Kennedy 1 , Katherine Drake 1 , Kristine Stump 1 , Lu Huo 1 , Yvonne Lo 1 , Min Ye 1 , Maryanne Covington 1 , Sharon Diamond 1 , Yan-Ou Yang 1 , Sunkyu Kim 1 , Swamy Yeleswaram 1 , Liangxing Wu 1 , Wenqing Yao 1
Affiliation
|
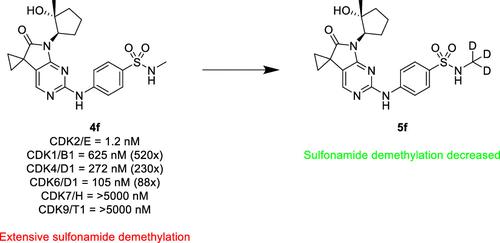
A series of exceptionally selective CDK2 inhibitors are described. Starting from an HTS hit, we successfully scaffold hopped to a 5,7-dihydro-6H-pyrrolo[2,3-d]pyrimidin-6-one core structure, which imparted a promising initial selectivity within the CDK family. Extensive further SAR identified additional factors that drove selectivity to above 200× for CDKs 1/4/6/7/9. General kinome selectivity was also greatly improved. Finally, use of in vivo metabolite identification allowed us to pinpoint sulfonamide dealkylation as the primary metabolite, which was ameliorated through the deuterium effect.
中文翻译:

发现 5,7-二氢-6H-吡咯并[2,3-d]嘧啶-6-酮作为高选择性 CDK2 抑制剂
描述了一系列具有特殊选择性的 CDK2 抑制剂。从HTS命中开始,我们成功地支架跳跃到5,7-二氢-6H-吡咯并[2,3- d ]嘧啶-6-一核心结构,这在CDK家族中赋予了有希望的初始选择性。进一步广泛的 SAR 确定了将 CDK 1/4/6/7/9 的选择性提高到 200 倍以上的其他因素。一般激酶组选择性也大大提高。最后,使用体内代谢物鉴定使我们能够将磺酰胺脱烷基化确定为主要代谢物,并通过氘效应得到改善。
更新日期:2022-10-06
中文翻译:

发现 5,7-二氢-6H-吡咯并[2,3-d]嘧啶-6-酮作为高选择性 CDK2 抑制剂
描述了一系列具有特殊选择性的 CDK2 抑制剂。从HTS命中开始,我们成功地支架跳跃到5,7-二氢-6H-吡咯并[2,3- d ]嘧啶-6-一核心结构,这在CDK家族中赋予了有希望的初始选择性。进一步广泛的 SAR 确定了将 CDK 1/4/6/7/9 的选择性提高到 200 倍以上的其他因素。一般激酶组选择性也大大提高。最后,使用体内代谢物鉴定使我们能够将磺酰胺脱烷基化确定为主要代谢物,并通过氘效应得到改善。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号