当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic effect-dependent intramolecular non-covalent interactions on the activity of 4,4-dimethylimidazolidin-2-one pharmacophore-based androgen receptor antagonists
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2022-10-05 , DOI: 10.1111/cbdd.14151 Muralikrishna Yaragani 1 , Prasad Yadlapalli 2 , Sriram Raghavan 3 , Thota Giridhar 4 , Venkata Basaveswara Rao Mandava 5 , Ravindra Vikram Singh 4 , Rajasekhara Prasad Kottapalli 1 , Saravanan Chinnusamy 6
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2022-10-05 , DOI: 10.1111/cbdd.14151 Muralikrishna Yaragani 1 , Prasad Yadlapalli 2 , Sriram Raghavan 3 , Thota Giridhar 4 , Venkata Basaveswara Rao Mandava 5 , Ravindra Vikram Singh 4 , Rajasekhara Prasad Kottapalli 1 , Saravanan Chinnusamy 6
Affiliation
|
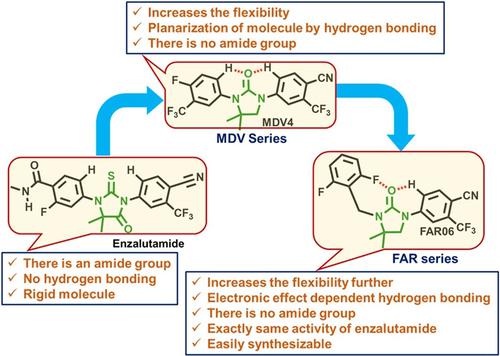
Because androgen receptor (AR) signalling is important for the development and progression of prostate cancer (PC), AR antagonists are utilized in clinical practices to treat PC and are referred to as androgen deprivation therapy (ADT). However, continued administration of AR antagonists often results in the development of resistance, known as castration-resistant prostate cancer (CRPC). Despite castration, it has been demonstrated that AR signalling continues to be fundamental to tumour growth. In this regard, a series of readily synthesizable 4,4-dimethylimidazolidine-2-one pharmacophore-based AR antagonists (FAR01-FAR11) were designed and synthesized. Androgen-dependent LNCaP PC cell line was used to test the AR-antagonist activity of these compounds in vitro and compared with the U.S. Food and Drug Administration (FDA) approved second-generation enzalutamide. In our previous work, rigid thiohydantoin pharmacophore in enzalutamide is replaced by the flexible 4,4-dimethylimidazolidin-2-one. In order to improve the flexibility further, one methylene group is introduced between the pharmacophore and one of the aromatic ring. Despite the fact that the amide functional group is a crucial characteristic for building AR antagonists, this class of molecules lacks one. FAR06 has the exact same activity as enzalutamide (IC50: 0.782 μM) with an IC50 value of 0.801 μM among the series of compounds.
中文翻译:

电子效应依赖性分子内非共价相互作用对 4,4-二甲基咪唑啉-2-一药效团雄激素受体拮抗剂活性的影响
由于雄激素受体 (AR) 信号对前列腺癌 (PC) 的发展和进展很重要,因此 AR 拮抗剂在临床实践中用于治疗 PC,并被称为雄激素剥夺疗法 (ADT)。然而,持续服用 AR 拮抗剂通常会导致耐药性的发展,称为去势抵抗性前列腺癌 (CRPC)。尽管进行了阉割,但已经证明 AR 信号仍然是肿瘤生长的基础。在这方面,一系列易于合成的基于 4,4-二甲基咪唑啉-2-酮药效团的 AR 拮抗剂(FAR01 - FAR11) 进行了设计和合成。雄激素依赖性 LNCaP PC 细胞系用于在体外测试这些化合物的 AR 拮抗剂活性,并与美国食品和药物管理局 (FDA) 批准的第二代恩杂鲁胺进行比较。在我们之前的工作中,enzalutamide 中的刚性乙内酰硫脲药效团被柔性的 4,4-二甲基咪唑啉-2-酮取代。为了进一步提高柔性,在药效团和芳环之一之间引入一个亚甲基。尽管酰胺官能团是构建 AR 拮抗剂的关键特征,但这类分子却缺少一个。FAR06具有与恩杂鲁胺(IC 50 :0.782 μM)完全相同的活性,IC 50该系列化合物中的值为 0.801 μM。
更新日期:2022-10-05
中文翻译:

电子效应依赖性分子内非共价相互作用对 4,4-二甲基咪唑啉-2-一药效团雄激素受体拮抗剂活性的影响
由于雄激素受体 (AR) 信号对前列腺癌 (PC) 的发展和进展很重要,因此 AR 拮抗剂在临床实践中用于治疗 PC,并被称为雄激素剥夺疗法 (ADT)。然而,持续服用 AR 拮抗剂通常会导致耐药性的发展,称为去势抵抗性前列腺癌 (CRPC)。尽管进行了阉割,但已经证明 AR 信号仍然是肿瘤生长的基础。在这方面,一系列易于合成的基于 4,4-二甲基咪唑啉-2-酮药效团的 AR 拮抗剂(FAR01 - FAR11) 进行了设计和合成。雄激素依赖性 LNCaP PC 细胞系用于在体外测试这些化合物的 AR 拮抗剂活性,并与美国食品和药物管理局 (FDA) 批准的第二代恩杂鲁胺进行比较。在我们之前的工作中,enzalutamide 中的刚性乙内酰硫脲药效团被柔性的 4,4-二甲基咪唑啉-2-酮取代。为了进一步提高柔性,在药效团和芳环之一之间引入一个亚甲基。尽管酰胺官能团是构建 AR 拮抗剂的关键特征,但这类分子却缺少一个。FAR06具有与恩杂鲁胺(IC 50 :0.782 μM)完全相同的活性,IC 50该系列化合物中的值为 0.801 μM。






























 京公网安备 11010802027423号
京公网安备 11010802027423号