当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Axially Chiral Biaryls via Enantioselective Ullmann Coupling of ortho-Chlorinated Aryl Aldehydes Enabled by a Chiral 2,2′-Bipyridine Ligand
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-10-04 , DOI: 10.1002/anie.202212108 Saima Perveen 1 , Shuai Zhang 2 , Linghua Wang 2 , Peidong Song 2 , Yizhao Ouyang 2 , Jiao Jiao 1 , Xin-Hua Duan 1 , Pengfei Li 1, 2, 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-10-04 , DOI: 10.1002/anie.202212108 Saima Perveen 1 , Shuai Zhang 2 , Linghua Wang 2 , Peidong Song 2 , Yizhao Ouyang 2 , Jiao Jiao 1 , Xin-Hua Duan 1 , Pengfei Li 1, 2, 3
Affiliation
|
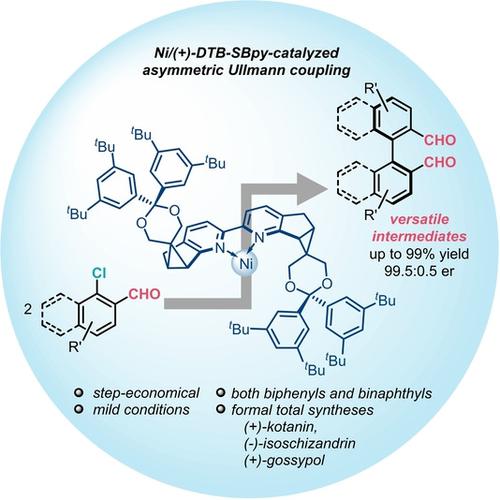
Reported is a general and highly enantioselective Ni-catalyzed reductive homocoupling of ortho-chlorinated aldehydes enabled by a SBpy-type chiral 2,2′-bipyridine ligand. This mild and step-economical approach allowed the synthesis of various axially chiral biaryl dials, and also provided versatile intermediates for diverse axially chiral catalysts, ligands, and natural products.
中文翻译:

通过手性 2,2'-联吡啶配体实现的邻氯代芳基醛的对映选择性 Ullmann 偶联合成轴向手性联芳基化合物
报道了由 SBpy 型手性 2,2'-联吡啶配体实现的一般且高度对映选择性的 Ni 催化的邻氯代醛的还原自偶联。这种温和且步骤经济的方法允许合成各种轴向手性联芳基表盘,并为各种轴向手性催化剂、配体和天然产物提供多功能中间体。
更新日期:2022-10-04
中文翻译:

通过手性 2,2'-联吡啶配体实现的邻氯代芳基醛的对映选择性 Ullmann 偶联合成轴向手性联芳基化合物
报道了由 SBpy 型手性 2,2'-联吡啶配体实现的一般且高度对映选择性的 Ni 催化的邻氯代醛的还原自偶联。这种温和且步骤经济的方法允许合成各种轴向手性联芳基表盘,并为各种轴向手性催化剂、配体和天然产物提供多功能中间体。































 京公网安备 11010802027423号
京公网安备 11010802027423号