当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mobility of Dissolved Gases in Smectites under Saturated Conditions: Effects of Pore Size, Gas Types, Temperature, and Surface Interaction
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-10-04 , DOI: 10.1021/acs.jpcc.2c05678 Jerry P. Owusu 1, 2 , Konstantinos Karalis 2 , Nikolaos I. Prasianakis 1 , Sergey V. Churakov 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2022-10-04 , DOI: 10.1021/acs.jpcc.2c05678 Jerry P. Owusu 1, 2 , Konstantinos Karalis 2 , Nikolaos I. Prasianakis 1 , Sergey V. Churakov 1, 2
Affiliation
|
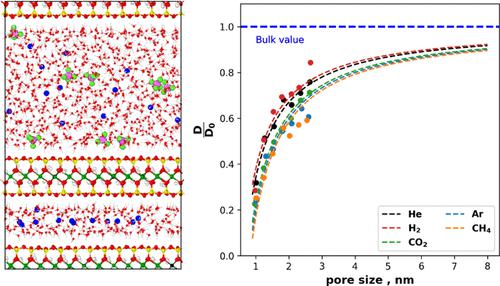
In a nuclear waste repository, the corrosion of metals and the degradation of the organic material in the waste matrix can generate significant amounts of gases. These gases should be able to migrate through the multibarrier system to prevent a potential pressure build-up that could lead to a loss of barrier integrity. Smectite mineral particles form a tortuous pore network consisting of larger interparticle pores and narrow interlayer pores between the platelets of the smectite minerals. These pores are normally saturated with water, so one of the most important mechanisms for the transport of gases is diffusion. The diffusion of gases through the interparticle porosity depends on the distribution of gas molecules in the water-rich phase, their self-diffusion coefficients, and the tortuosity of the pore space. Classical molecular dynamics simulations were applied to study the mobility of gases (CO2, H2, CH4, He, and Ar) in Na-montmorillonite (Na-MMT) under saturated conditions. The simulations were used to estimate the gas diffusion coefficient (D) in saturated Na-MMT as a function of nanopore size and temperature. The temperature dependence of the diffusion coefficient was expressed by the Arrhenius equation for the activation energy (Ea). The predicted D values of gases were found to be sensitive to the pore size as the D values gradually increase with increasing pore size and asymptotically converge to the gas diffusion coefficient in bulk water. This behavior is also observed in the self-diffusion coefficients of water in Na-MMT. In general, H2 and He exhibit higher D values than Ar, CO2, and CH4. The predicted Ea values indicate that the confinement affects the activation energy. This effect is due to the structuring of the water molecules near the clay surface, which is more pronounced in the first two layers of water near the surface and decreases thereafter. Atomic density profiles and radial distribution functions obtained from the simulations show that the interaction of the gas with the liquid and the clay surface influences mobility. The obtained diffusion coefficient for different gases and slit pore size were parameterized with a single empirical relationship, which can be applied to macroscopic simulations of gas transport.
中文翻译:

饱和条件下蒙脱石中溶解气体的迁移率:孔径、气体类型、温度和表面相互作用的影响
在核废料储存库中,金属腐蚀和废料基质中有机材料的降解会产生大量气体。这些气体应该能够通过多屏障系统迁移,以防止可能导致屏障完整性丧失的潜在压力积聚。蒙脱石矿物颗粒形成曲折的孔隙网络,由较大的颗粒间孔隙和蒙脱石矿物片晶之间的狭窄层间孔隙组成。这些孔隙通常被水饱和,因此气体传输的最重要机制之一是扩散。气体通过颗粒间孔隙的扩散取决于气体分子在富水相中的分布、它们的自扩散系数和孔隙空间的曲折度。2 , H 2 , CH 4 , He, 和 Ar) 在饱和条件下在 Na-蒙脱石 (Na-MMT) 中。模拟用于估计饱和 Na-MMT 中的气体扩散系数 ( D ) 作为纳米孔径和温度的函数。扩散系数的温度依赖性由活化能 ( E a ) 的 Arrhenius 方程表示。发现气体的预测D值对孔径敏感,因为D值随着孔径的增加而逐渐增加,并渐近收敛于散装水中的气体扩散系数。在 Na-MMT 中水的自扩散系数中也观察到了这种行为。一般来说,H2和He表现出比Ar、CO 2和CH 4更高的D值。预测的E a值表明约束影响活化能。这种效应是由于粘土表面附近的水分子的结构化,在靠近表面的前两层水中更为明显,此后逐渐减少。从模拟中获得的原子密度分布和径向分布函数表明,气体与液体和粘土表面的相互作用会影响流动性。所获得的不同气体的扩散系数和狭缝孔径采用单一的经验关系进行参数化,可应用于气体传输的宏观模拟。
更新日期:2022-10-04
中文翻译:

饱和条件下蒙脱石中溶解气体的迁移率:孔径、气体类型、温度和表面相互作用的影响
在核废料储存库中,金属腐蚀和废料基质中有机材料的降解会产生大量气体。这些气体应该能够通过多屏障系统迁移,以防止可能导致屏障完整性丧失的潜在压力积聚。蒙脱石矿物颗粒形成曲折的孔隙网络,由较大的颗粒间孔隙和蒙脱石矿物片晶之间的狭窄层间孔隙组成。这些孔隙通常被水饱和,因此气体传输的最重要机制之一是扩散。气体通过颗粒间孔隙的扩散取决于气体分子在富水相中的分布、它们的自扩散系数和孔隙空间的曲折度。2 , H 2 , CH 4 , He, 和 Ar) 在饱和条件下在 Na-蒙脱石 (Na-MMT) 中。模拟用于估计饱和 Na-MMT 中的气体扩散系数 ( D ) 作为纳米孔径和温度的函数。扩散系数的温度依赖性由活化能 ( E a ) 的 Arrhenius 方程表示。发现气体的预测D值对孔径敏感,因为D值随着孔径的增加而逐渐增加,并渐近收敛于散装水中的气体扩散系数。在 Na-MMT 中水的自扩散系数中也观察到了这种行为。一般来说,H2和He表现出比Ar、CO 2和CH 4更高的D值。预测的E a值表明约束影响活化能。这种效应是由于粘土表面附近的水分子的结构化,在靠近表面的前两层水中更为明显,此后逐渐减少。从模拟中获得的原子密度分布和径向分布函数表明,气体与液体和粘土表面的相互作用会影响流动性。所获得的不同气体的扩散系数和狭缝孔径采用单一的经验关系进行参数化,可应用于气体传输的宏观模拟。






























 京公网安备 11010802027423号
京公网安备 11010802027423号