当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(I)-Catalyzed Tandem Cyclization/Hydroarylation of o-Alkynylphenols with Haloalkynes
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-10-04 , DOI: 10.1021/acs.joc.2c01804
Jiawen Wu 1 , Cunbo Wei 1 , Fen Zhao 2 , Wenqian Du 1 , Zhishuai Geng 1 , Zhonghua Xia 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-10-04 , DOI: 10.1021/acs.joc.2c01804
Jiawen Wu 1 , Cunbo Wei 1 , Fen Zhao 2 , Wenqian Du 1 , Zhishuai Geng 1 , Zhonghua Xia 1
Affiliation
|
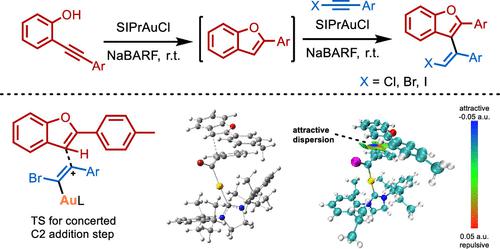
A convenient and mild protocol for the gold-catalyzed intermolecular coupling of o-alkynylphenols with haloalkynes to give vinyl benzofurans is reported. In this work, the gold catalyst SIPrAuCl and the co-catalyst NaBARF would corporately promote the intramolecular cyclization of the o-alkynylphenol to benzofuran, and then a selective hydroarylation of benzofuran to haloalkyne was catalyzed by the same catalysts. Computational studies suggest that the hydroarylation process takes place via a concerted nucleophilic attack pathway of the benzofuran to the C2 carbon of the activated haloalkyne, and reveal the original driving force of this hydroarylation process.
中文翻译:

金 (I)-催化邻炔基苯酚与卤代炔烃的串联环化/氢化
报道了一种方便且温和的方案,用于金催化邻炔基苯酚与卤代炔烃的分子间偶联,得到乙烯基苯并呋喃。在这项工作中,金催化剂 SIPrAuCl 和助催化剂 NaBARF 将共同促进邻炔基苯酚分子内环化为苯并呋喃,然后由相同催化剂催化苯并呋喃选择性加氢芳基化为卤代炔烃。计算研究表明,加氢芳基化过程是通过苯并呋喃对活化卤代炔烃的 C2 碳的协同亲核攻击途径发生的,并揭示了该加氢芳基化过程的原始驱动力。
更新日期:2022-10-04
中文翻译:

金 (I)-催化邻炔基苯酚与卤代炔烃的串联环化/氢化
报道了一种方便且温和的方案,用于金催化邻炔基苯酚与卤代炔烃的分子间偶联,得到乙烯基苯并呋喃。在这项工作中,金催化剂 SIPrAuCl 和助催化剂 NaBARF 将共同促进邻炔基苯酚分子内环化为苯并呋喃,然后由相同催化剂催化苯并呋喃选择性加氢芳基化为卤代炔烃。计算研究表明,加氢芳基化过程是通过苯并呋喃对活化卤代炔烃的 C2 碳的协同亲核攻击途径发生的,并揭示了该加氢芳基化过程的原始驱动力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号