当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Simple Estimate of the Potential Drop across an Amphiprotic Liquid–Liquid Interface
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2022-10-04 , DOI: 10.1021/acs.jpcb.2c05696 Christian F Chamberlayne 1 , Richard N Zare 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2022-10-04 , DOI: 10.1021/acs.jpcb.2c05696 Christian F Chamberlayne 1 , Richard N Zare 1
Affiliation
|
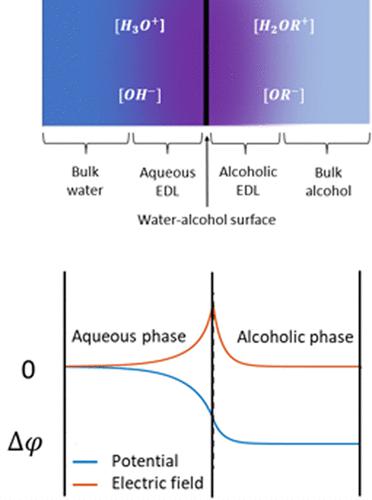
Two immiscible liquids in contact with each other can have different internal electrostatic potentials. An associated electric double layer (EDL) therefore exists within each liquid. For amphiprotic liquids, the exchange of protons between the two liquids gives rise to two EDLs, a positively charged EDL in one of the liquids and negatively charged EDL in the other. Using the pKa and pKb of one liquid dissolved in the other and the pH equivalent within each amphiprotic liquid, we can estimate the potential drop, Δφ, between the interior of the two liquids, also known as the Galvani potential or liquid–liquid junction potential. This estimation is independent of surface charge and ionic strength. By using the ionic strength to find the thickness of the EDL, we also estimate the average electric field strength across the interface. For the special case of water (H2O) in contact with an immiscible alcohol (ROH), the potential drop across the interface from the water to the alcohol is Δφ = 2.303VT (pKb + pH – pKw – pH2OR), where VT is the thermal voltage at a given temperature T.
中文翻译:

两性液-液界面电位降的简单估计
相互接触的两种不混溶液体可能具有不同的内部静电势。因此,每种液体内存在相关联的双电层 (EDL)。对于两性液体,两种液体之间的质子交换产生两种 EDL,一种液体中带正电的 EDL,另一种液体中带负电的 EDL。使用一种液体溶解在另一种液体中的 p K a和 p K b以及每种两性液体中的 pH 值,我们可以估算电位降 Δ φ,在两种液体的内部之间,也称为 Galvani 势或液-液接界势。该估计与表面电荷和离子强度无关。通过使用离子强度来确定 EDL 的厚度,我们还可以估算界面上的平均电场强度。对于水 (H 2 O) 与不混溶的酒精 (ROH) 接触的特殊情况,从水到酒精的界面电位降为 Δ φ = 2.303 V T ( pK b + pH – pK w – pH 2或), 其中V T是给定温度T下的热电压。
更新日期:2022-10-04
中文翻译:

两性液-液界面电位降的简单估计
相互接触的两种不混溶液体可能具有不同的内部静电势。因此,每种液体内存在相关联的双电层 (EDL)。对于两性液体,两种液体之间的质子交换产生两种 EDL,一种液体中带正电的 EDL,另一种液体中带负电的 EDL。使用一种液体溶解在另一种液体中的 p K a和 p K b以及每种两性液体中的 pH 值,我们可以估算电位降 Δ φ,在两种液体的内部之间,也称为 Galvani 势或液-液接界势。该估计与表面电荷和离子强度无关。通过使用离子强度来确定 EDL 的厚度,我们还可以估算界面上的平均电场强度。对于水 (H 2 O) 与不混溶的酒精 (ROH) 接触的特殊情况,从水到酒精的界面电位降为 Δ φ = 2.303 V T ( pK b + pH – pK w – pH 2或), 其中V T是给定温度T下的热电压。































 京公网安备 11010802027423号
京公网安备 11010802027423号