当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 Hydrogenation to Methanol on Indium Oxide-Supported Rhenium Catalysts: The Effects of Size
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-04 , DOI: 10.1021/acscatal.2c03709 Chenyang Shen 1, 2 , Kaihang Sun 1 , Rui Zou 1, 2 , Qinglei Wu 1, 2 , Donghai Mei 3 , Chang-jun Liu 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-10-04 , DOI: 10.1021/acscatal.2c03709 Chenyang Shen 1, 2 , Kaihang Sun 1 , Rui Zou 1, 2 , Qinglei Wu 1, 2 , Donghai Mei 3 , Chang-jun Liu 1, 2
Affiliation
|
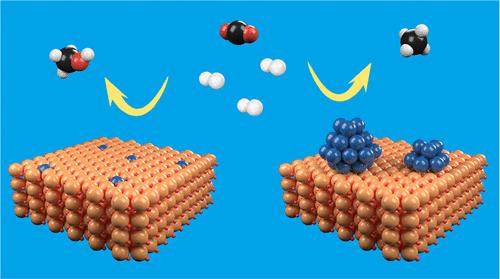
In this work, CO2 hydrogenation over In2O3-supported rhenium (Re) catalysts was found to be highly size-dependent. When the Re loading was less than 1 wt %, the strong interaction between Re and In2O3 caused atomically dispersed Re species with a positive charge, resulting in high activity for CO2 hydrogenation to methanol with enhanced stability at elevated temperatures. The space–time yield of methanol over the 1 wt % Re/In2O3 catalyst reached 0.54 gMeOH gcat–1 h–1 with a methanol selectivity of 72.1% at 5 MPa and 573 K. With increasing Re loading, the In2O3 supported Re catalysts become more favored for CO2 methanation. Under the same experimental conditions, the methane selectivity is close to 100.0% over the 10 wt % Re/In2O3 catalyst. Catalyst characterizations and density functional theoretical (DFT) calculations further confirm that the size of the Re/In2O3 catalyst has a significant effect on hydrogen activation and the selectivity of the CO2 hydrogenation reaction. Due to the strong Re–In2O3 interaction, the atomically dispersed Re in the In2O3 surface lattice not only stabilizes oxygen vacancies but also results in Hδ+ formation upon hydrogen adsorption. This significantly promotes methanol synthesis from CO2 hydrogenation. Meanwhile, the 10 wt % Re/In2O3 catalyst with supported Re nanoclusters induces Hδ- formation, which eventually leads to more methane production. The present study demonstrates the atomically dispersed Re/In2O3 catalyst is promising for CO2 hydrogenation to methanol.
中文翻译:

氧化铟负载铼催化剂上 CO2 加氢制甲醇:尺寸的影响
在这项工作中,发现在 In 2 O 3负载的铼 (Re) 催化剂上的CO 2加氢高度依赖于尺寸。当 Re 负载量小于 1 wt% 时,Re 和 In 2 O 3之间的强相互作用导致具有正电荷的原子分散的 Re 物种,导致 CO 2加氢制甲醇的高活性,并在高温下增强稳定性。在 1 wt % Re/In 2 O 3催化剂上甲醇的时空产率达到 0.54 g MeOH g cat –1 h –1在 5 MPa 和 573 K 条件下甲醇选择性为 72.1%。随着 Re 负载量的增加,In 2 O 3负载的 Re 催化剂对 CO 2甲烷化变得更有利。在相同的实验条件下,10 wt% Re/In 2 O 3催化剂的甲烷选择性接近100.0% 。催化剂表征和密度泛函理论(DFT)计算进一步证实了Re/In 2 O 3催化剂的尺寸对氢气活化和CO 2加氢反应的选择性有显着影响。由于强 Re-In 2 O 3相互作用,In 2 O 3表面晶格中原子分散的 Re 不仅稳定了氧空位,而且在氢吸附时导致 H δ+的形成。这显着促进了由CO 2加氢合成甲醇。同时,负载有 Re 纳米团簇的 10 wt% Re/In 2 O 3催化剂诱导 H δ -的形成,最终导致更多的甲烷产生。本研究表明原子分散的 Re/In 2 O 3催化剂有望用于 CO 2加氢制甲醇。
更新日期:2022-10-04
中文翻译:

氧化铟负载铼催化剂上 CO2 加氢制甲醇:尺寸的影响
在这项工作中,发现在 In 2 O 3负载的铼 (Re) 催化剂上的CO 2加氢高度依赖于尺寸。当 Re 负载量小于 1 wt% 时,Re 和 In 2 O 3之间的强相互作用导致具有正电荷的原子分散的 Re 物种,导致 CO 2加氢制甲醇的高活性,并在高温下增强稳定性。在 1 wt % Re/In 2 O 3催化剂上甲醇的时空产率达到 0.54 g MeOH g cat –1 h –1在 5 MPa 和 573 K 条件下甲醇选择性为 72.1%。随着 Re 负载量的增加,In 2 O 3负载的 Re 催化剂对 CO 2甲烷化变得更有利。在相同的实验条件下,10 wt% Re/In 2 O 3催化剂的甲烷选择性接近100.0% 。催化剂表征和密度泛函理论(DFT)计算进一步证实了Re/In 2 O 3催化剂的尺寸对氢气活化和CO 2加氢反应的选择性有显着影响。由于强 Re-In 2 O 3相互作用,In 2 O 3表面晶格中原子分散的 Re 不仅稳定了氧空位,而且在氢吸附时导致 H δ+的形成。这显着促进了由CO 2加氢合成甲醇。同时,负载有 Re 纳米团簇的 10 wt% Re/In 2 O 3催化剂诱导 H δ -的形成,最终导致更多的甲烷产生。本研究表明原子分散的 Re/In 2 O 3催化剂有望用于 CO 2加氢制甲醇。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号