当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controllable Cycloadditions between 2H-(Thio)pyran-2-(thi)ones and Strained Alkynes: A Click-and-Release Strategy for COS/H2S Generation
Organic Letters ( IF 4.9 ) Pub Date : 2022-10-03 , DOI: 10.1021/acs.orglett.2c02819 Qi Cui 1 , Tony W Pan 1 , Meg Shieh 1 , Shane S Kelly 2 , Shi Xu 1 , Wei-Jun Qian 2 , Ming Xian 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-10-03 , DOI: 10.1021/acs.orglett.2c02819 Qi Cui 1 , Tony W Pan 1 , Meg Shieh 1 , Shane S Kelly 2 , Shi Xu 1 , Wei-Jun Qian 2 , Ming Xian 1
Affiliation
|
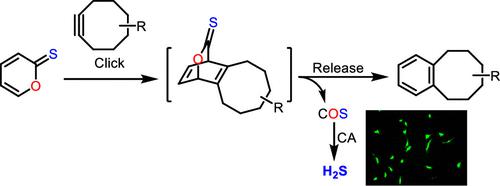
In this work, we carried out computational studies to predict the cycloaddition efficiency of strained alkynes with 2H-pyran-2-one and its three sulfur-containing analogues: 2H-pyran-2-thione, 2H-thiopyran-2-one, and 2H-thiopyran-2-thione. It was predicted that the decreased aromaticity of the substrate would yield higher reactivity. Experimental studies confirmed the calculation results, and 2H-pyan-2-thiones were found to be the most reactive substrates. This reaction proceeded effectively in aqueous buffers and in cellular environments. It also produced COS as the byproduct, which could be converted into hydrogen sulfide (H2S) in the presence of carbonate anhydrase. This click-and-release approach may serve as a unique way to deliver COS/H2S to specific locations.
中文翻译:

2H-(Thio)pyran-2-(thi)ones 和应变炔烃之间的可控环加成:COS/H2S 生成的点击释放策略
在这项工作中,我们进行了计算研究,以预测应变炔烃与 2 H -pyran-2-one 及其三种含硫类似物:2 H -pyran-2-thione、2 H -thiopyran-2-的环加成效率一,和 2 H -thiopyran-2-thione。据预测,底物的芳香性降低会产生更高的反应性。实验研究证实了计算结果,发现 2 H -pyan-2-thiones 是最具反应性的底物。该反应在水性缓冲液和细胞环境中有效进行。它还产生副产品COS,可转化为硫化氢(H 2S) 在碳酸酐酶存在下。这种点击和释放方法可以作为将 COS/H 2 S 输送到特定位置的独特方式。
更新日期:2022-10-03
中文翻译:

2H-(Thio)pyran-2-(thi)ones 和应变炔烃之间的可控环加成:COS/H2S 生成的点击释放策略
在这项工作中,我们进行了计算研究,以预测应变炔烃与 2 H -pyran-2-one 及其三种含硫类似物:2 H -pyran-2-thione、2 H -thiopyran-2-的环加成效率一,和 2 H -thiopyran-2-thione。据预测,底物的芳香性降低会产生更高的反应性。实验研究证实了计算结果,发现 2 H -pyan-2-thiones 是最具反应性的底物。该反应在水性缓冲液和细胞环境中有效进行。它还产生副产品COS,可转化为硫化氢(H 2S) 在碳酸酐酶存在下。这种点击和释放方法可以作为将 COS/H 2 S 输送到特定位置的独特方式。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号