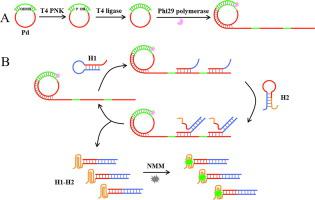
T4 多核苷酸激酶 (PNK) 在维持基因组完整性和修复 DNA 损伤方面起着关键作用。在本文中,我们提出了一种基于滚环扩增 (RCA) 和催化发夹组装 (CHA) 的 T4 PNK 活性放大检测的无标记荧光生物传感器。首先,我们设计了一个挂锁探针,其具有用于磷酸化反应的 5'-羟基末端、用于启动 RCA 的引物互补序列和用于触发 CHA 的触发器互补序列。T4 PNK通过在挂锁探针的5'-羟基末端添加磷酸基团来催化磷酸化反应,生成磷酸化的挂锁探针。然后在连接酶的作用下与引物杂交生成环状探针。随后,引物沿着环状探针引发RCA反应,合成具有重复触发序列的大分子量产物。然后触发器触发发夹探针 1 和发夹探针 2 之间的循环组装反应,以生成大量具有游离的富含 G 序列的复合物。游离的富含 G 的序列折叠成 G-四链体结构,并将 N-甲基中卟啉 IX 插入其中以产生放大的荧光信号。得益于 RCA 和 CHA 的高扩增效率,该荧光生物传感器可以检测低至 6.63×10 的 T4 PNK 游离的富含 G 的序列折叠成 G-四链体结构,并将 N-甲基中卟啉 IX 插入其中以产生放大的荧光信号。得益于 RCA 和 CHA 的高扩增效率,该荧光生物传感器可以检测低至 6.63×10 的 T4 PNK 游离的富含 G 的序列折叠成 G-四链体结构,并将 N-甲基中卟啉 IX 插入其中以产生放大的荧光信号。得益于 RCA 和 CHA 的高扩增效率,该荧光生物传感器可以检测低至 6.63×10 的 T4 PNK-4 U mL -1,并成功应用于检测其在HeLa细胞裂解液中的活性。此外,该荧光生物传感器可有效区分T4 PNK与其他替代物,并评估抑制剂的抑制作用,表明其在药物筛选和疾病治疗方面具有巨大潜力。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
A label-free fluorescent biosensor for amplified detection of T4 polynucleotide kinase activity based on rolling circle amplification and catalytic hairpin assembly
T4 polynucleotide kinase (PNK) plays a key role in maintaining genome integrity and repairing DNA damage. In this paper, we proposed a label-free fluorescent biosensor for amplified detection of T4 PNK activity based on rolling circle amplification (RCA) and catalytic hairpin assembly (CHA). Firstly, we designed a padlock probe with a 5′-hydroxyl terminus for phosphorylation reaction, a complementary sequence of the primer for initiating RCA, and a complementary sequence of the trigger for triggering CHA. T4 PNK catalyzed the phosphorylation reaction by adding a phosphate group to the 5′-hydroxyl terminus of padlock probe, generating a phosphorylated padlock probe. Then it hybridized with the primer to generate a circular probe under the action of ligase. Subsequently, the primer initiated an RCA reaction along the circular probe to synthesize a large molecular weight product with repetitive trigger sequences. The triggers then triggered the cyclic assembly reactions between hairpin probe 1 and hairpin probe 2 to generate a large amount of complexes with free G-rich sequences. The free G-rich sequences folded into G-quadruplex structures, and the N-methylmesoporphyrin IXs were inserted into them to produce an amplified fluorescent signal. Benefiting from high amplification efficiency of RCA and CHA, this fluorescent biosensor could detect T4 PNK as low as 6.63×10-4 U mL-1, and was successfully applied to detect its activity in HeLa cell lysates. Moreover, this fluorescent biosensor could effectively distinguish T4 PNK from other alternatives and evaluate the inhibitory effect of inhibitor, indicating that it had great potential in drug screening and disease treatment.
































 京公网安备 11010802027423号
京公网安备 11010802027423号