当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium‐Catalyzed Dynamic Kinetic Asymmetric Allylation of Phenols and 2‐Hydroxypyridines
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-09-13 , DOI: 10.1002/chem.201603532 Changkun Li 1 , Bernhard Breit 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2016-09-13 , DOI: 10.1002/chem.201603532 Changkun Li 1 , Bernhard Breit 1
Affiliation
|
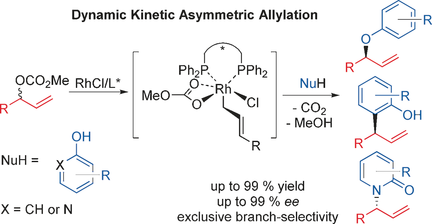
Inspired by the mechanistic studies of rhodium‐catalyzed atom‐economic addition of carboxylate acids to allenes, a rhodium‐catalyzed dynamic kinetic asymmetric allylation of different nucleophiles with racemic allylic carbonates has been developed. High regio‐ and enantioselectivities can be obtained under neutral conditions and, furthermore, the chemoselectivities can be controlled by different diphosphine ligands. (R,R)‐QuinoxP* leads to selective O‐allylation of phenols, whereas when embedding (S,S)‐DIOP as the ligand, 2‐naphthol is ortho‐C‐allylated for the first time in high enantioselectivity. To this end, hydroxypyridines can be N‐allylated by RhI/(S)‐DTBM‐Segphos via the same intermediate as in the previously reported atom‐economic addition to allenes.
中文翻译:

铑催化的苯酚和2-羟基吡啶的动力学动力学不对称烯丙基化
受铑催化羧酸经济向丙二烯中添加羧酸的机理研究的启发,已开发出铑催化的不同亲核试剂与消旋烯丙基碳酸酯的动态动力学不对称烯丙基化反应。在中性条件下可以获得很高的区域选择性和对映选择性,此外,化学选择性可以通过不同的二膦配体来控制。(R,R)-QuinoxP *导致酚的选择性O-烯丙基化,而当嵌入(S,S)-DIOP作为配体时,2-萘酚首次以高对映选择性被邻-C-烯丙基化。为此,羟基吡啶可以通过Rh I /(S)-DTBM-Segphos的中间体与先前报道的对丙二烯的原子经济加成反应相同。
更新日期:2016-09-13
中文翻译:

铑催化的苯酚和2-羟基吡啶的动力学动力学不对称烯丙基化
受铑催化羧酸经济向丙二烯中添加羧酸的机理研究的启发,已开发出铑催化的不同亲核试剂与消旋烯丙基碳酸酯的动态动力学不对称烯丙基化反应。在中性条件下可以获得很高的区域选择性和对映选择性,此外,化学选择性可以通过不同的二膦配体来控制。(R,R)-QuinoxP *导致酚的选择性O-烯丙基化,而当嵌入(S,S)-DIOP作为配体时,2-萘酚首次以高对映选择性被邻-C-烯丙基化。为此,羟基吡啶可以通过Rh I /(S)-DTBM-Segphos的中间体与先前报道的对丙二烯的原子经济加成反应相同。































 京公网安备 11010802027423号
京公网安备 11010802027423号