当前位置:
X-MOL 学术
›
Adv. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Unimolecular System Combining Efficient Inhibition of NDM-1 by Coordination Interactions with High ROS for Synergistically Tackling Drug-Resistant Bacteria
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2022-09-30 , DOI: 10.1002/admi.202201329
Meiqi Li 1 , Ling li 1 , Qiong Yuan 1 , Benkai Bao 1 , Yanli Tang 1
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2022-09-30 , DOI: 10.1002/admi.202201329
Meiqi Li 1 , Ling li 1 , Qiong Yuan 1 , Benkai Bao 1 , Yanli Tang 1
Affiliation
|
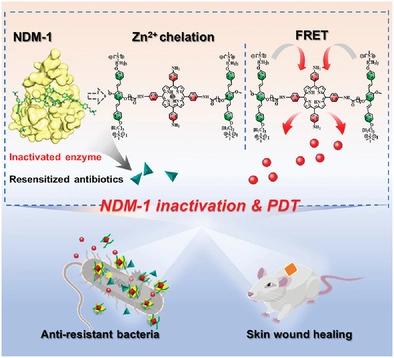
New Delhi metallo-β-lactamase (NDM-1) that can hydrolyze almost all clinical β-lactam antibacterial causes “Super” bacterial infection, which seriously threatens human health worldwide. Therefore, it is urgent to establish powerful methods for inhibiting NDM-1 activity and efficient anti-resistant bacteria. Herein, a new strategy for the depression of NDM-1 activity and synergistic therapy to overcome drug-resistant bacteria based on conjugated oligomer (OPV)-modified porphyrin derivatives OPV-C6-TPP probe is developed. The unimolecular probe not only can coordinate Zn2+ in NDM-1 to suppress the activity of NDM-1 but also produce the high 1O2 under light irradiation, providing dual functions of activating antibiotics and photodynamic therapy. The chelation inhibition mechanism of OPV-C6-TPP on NDM-1 is investigated and further confirmed by molecular docking and molecular dynamics simulation studies. In the presence of probe, the minimum inhibitory concentration of antibiotic model cefazolin is reduced ≈50 times, significantly reversing resistance and resensitizing NDM-1-overexpressing Escherichia coli (E. coli-NDM-1) to antibiotics. Furthermore, the combined system greatly accelerates wound healing in E. coli-NDM-1 infected mice due to photodynamic and antibiotic synergistic effects. This study paves the way for the efficient treatment of drug-resistant bacteria.
中文翻译:

一种通过配位相互作用有效抑制 NDM-1 的单分子系统与高 ROS 协同处理耐药细菌
新德里金属-β-内酰胺酶(NDM-1)能水解几乎所有临床上的β-内酰胺类抗菌药物,造成“超级”细菌感染,严重威胁全球人类健康。因此,迫切需要建立抑制NDM-1活性的有效方法和高效的抗耐药菌。在此,开发了一种基于共轭低聚物 (OPV) 修饰的卟啉衍生物 OPV-C 6 -TPP 探针的抑制 NDM-1 活性和协同治疗以克服耐药细菌的新策略。该单分子探针不仅可以配位NDM-1中的Zn 2+抑制NDM-1的活性,还可以产生高的1 O 2在光照射下,提供激活抗生素和光动力疗法的双重功能。通过分子对接和分子动力学模拟研究进一步证实了OPV-C 6 -TPP对NDM-1的螯合抑制机制。在探针存在的情况下,抗生素模型头孢唑林的最低抑菌浓度降低≈50倍,显着逆转耐药性并使过表达NDM-1的大肠杆菌(E. coli -NDM-1)对抗生素重新敏感。此外,由于光动力和抗生素的协同作用,组合系统大大加速了大肠杆菌-NDM-1 感染小鼠的伤口愈合。该研究为有效治疗耐药菌铺平了道路。
更新日期:2022-09-30
中文翻译:

一种通过配位相互作用有效抑制 NDM-1 的单分子系统与高 ROS 协同处理耐药细菌
新德里金属-β-内酰胺酶(NDM-1)能水解几乎所有临床上的β-内酰胺类抗菌药物,造成“超级”细菌感染,严重威胁全球人类健康。因此,迫切需要建立抑制NDM-1活性的有效方法和高效的抗耐药菌。在此,开发了一种基于共轭低聚物 (OPV) 修饰的卟啉衍生物 OPV-C 6 -TPP 探针的抑制 NDM-1 活性和协同治疗以克服耐药细菌的新策略。该单分子探针不仅可以配位NDM-1中的Zn 2+抑制NDM-1的活性,还可以产生高的1 O 2在光照射下,提供激活抗生素和光动力疗法的双重功能。通过分子对接和分子动力学模拟研究进一步证实了OPV-C 6 -TPP对NDM-1的螯合抑制机制。在探针存在的情况下,抗生素模型头孢唑林的最低抑菌浓度降低≈50倍,显着逆转耐药性并使过表达NDM-1的大肠杆菌(E. coli -NDM-1)对抗生素重新敏感。此外,由于光动力和抗生素的协同作用,组合系统大大加速了大肠杆菌-NDM-1 感染小鼠的伤口愈合。该研究为有效治疗耐药菌铺平了道路。

































 京公网安备 11010802027423号
京公网安备 11010802027423号