Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2022-10-01 , DOI: 10.1016/j.jpba.2022.115089 Hanno Stutz 1
|
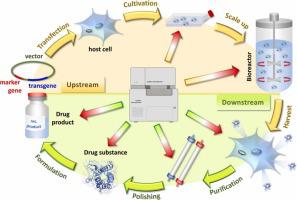
This review provides a comprehensive overview of methodological advances and applications of CE in the analysis and characterization of recombinant therapeutic and diagnostic proteins over the past two decades. The first part of the review discusses various aspects of biotechnological protein production and the related effects on the final product. This covers upstream processes, e.g., selection and transfection of host cells, up-scaling of cell cultures and cultivation conditions, as well as downstream processing and a discussion of future trends in biotechnological manufacturing. This part is essential for relating biotechnological production to analytical challenges and requirements in order to provide a holistic insight. In this context, the influence of manufacturing steps on the quality of the final drug substance/product is discussed in terms of related post-translational modifications of the target molecule with a major focus on glycosylation pattern and conformational effects. Particular attention is given to host cell specific and non-human modifications affecting the efficacy and safety of recombinant products. Endowed with this propaedeutic knowledge, the major part of the review discusses the manifold contributions of different CE techniques to the development and optimization of the manufacturing process, to the evaluation and characterization of the final drug product and their role in quality control. Different CE techniques, such as CZE, capillary gel electrophoresis (CGE), (imaged) capillary isoelectric focusing ((i)CIEF), µChipCE, CE-Western blot, affinity CE (ACE), and CE-MS are discussed including a brief introduction in the respective separation and hyphenation principle as well as their applications in the analysis of different recombinant biologics together with recent strategies. The addressed analyte portfolio comprises a vast variety of recombinant proteins with molecular masses from 4.1 kDa up to 20.3 MDa (for recombinant virus-like particles), and a pI range from 2.0 to 11.2. Antibodies are not explicitly covered in the survey. The review is complemented by compiling validation aspects and proposed suitability tests in order to assure the feasibility of methods to industrial and pharmaceutical needs.
中文翻译:

毛细管电迁移方法在治疗性和诊断性重组蛋白分析中的进展和应用——综述
本综述全面概述了过去二十年来 CE 在重组治疗和诊断蛋白的分析和表征中的方法学进展和应用。评论的第一部分讨论了生物技术蛋白质生产的各个方面以及对最终产品的相关影响。这包括上游过程,例如宿主细胞的选择和转染,细胞培养和培养条件的放大,以及下游加工和对生物技术制造未来趋势的讨论。这部分对于将生物技术生产与分析挑战和要求联系起来以提供整体洞察力至关重要。在这种情况下,制造步骤对最终原料药/产品质量的影响根据目标分子的相关翻译后修饰进行了讨论,主要关注糖基化模式和构象效应。特别关注影响重组产品功效和安全性的宿主细胞特异性和非人类修饰。有了这些基础知识,本综述的主要部分讨论了不同 CE 技术对生产过程的开发和优化、最终药物产品的评估和表征及其在质量控制中的作用的多方面贡献。不同的 CE 技术,例如 CZE、毛细管凝胶电泳 (CGE)、(成像)毛细管等电聚焦 ((i)CIEF)、µChipCE、CE-Western 印迹、亲和 CE (ACE)、讨论了 CE-MS 和 CE-MS,包括简要介绍各自的分离和连字原理以及它们在分析不同重组生物制剂中的应用以及最近的策略。所涉及的分析物组合包括分子量从 4.1 kDa 到高达 20.3 MDa(对于重组病毒样颗粒)和 pI 范围从 2.0 到 11.2 的各种重组蛋白。该调查未明确涵盖抗体。审查通过汇编验证方面和建议的适用性测试来补充,以确保方法对工业和制药需求的可行性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号