当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
General and Scalable Approach to A2B- and A2BC-Type Porphyrin Phosphonate Diesters
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-10-01 , DOI: 10.1002/ejoc.201600857
Yulia Yu. Enakieva 1, 2 , Julien Michalak 1 , Inna A. Abdulaeva 1, 2 , Marina V. Volostnykh 1, 2 , Christine Stern 1 , Roger Guilard 1 , Alla G. Bessmertnykh-Lemeune 1 , Yulia G. Gorbunova 2, 3 , Aslan Yu. Tsivadze 3 , Karl M. Kadish 4
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2016-10-01 , DOI: 10.1002/ejoc.201600857
Yulia Yu. Enakieva 1, 2 , Julien Michalak 1 , Inna A. Abdulaeva 1, 2 , Marina V. Volostnykh 1, 2 , Christine Stern 1 , Roger Guilard 1 , Alla G. Bessmertnykh-Lemeune 1 , Yulia G. Gorbunova 2, 3 , Aslan Yu. Tsivadze 3 , Karl M. Kadish 4
Affiliation
|
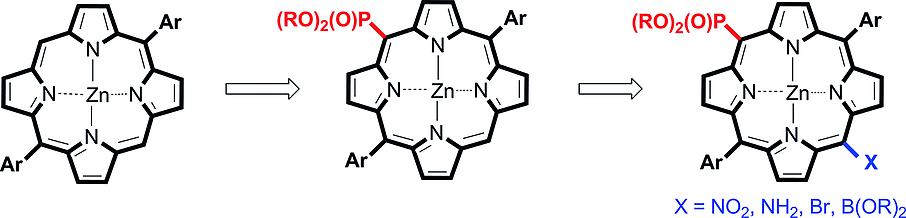
A two-step reaction sequence for accessing meso-(dialkoxyphosphoryl)porphyrins from readily available trans-A2-type porphyrins was developed. This approach involves bromination and subsequent palladium-catalyzed phosphonylation. Optimal conditions for both steps were identified after exploration of various reaction parameters such as solvent, temperature and catalyst. A series of dialkoxyphosphoryl-substituted A2B-porphyrins Zn3(a–g) bearing electron-donating, electron-withdrawing or sterically bulky substituents at the meso-aryl groups were prepared in overall yields close to 40 %. These compounds, being air-stable and soluble in most organic solvents, are valuable synthetic intermediates because they can be readily transformed into functionalized trans-A2BC-type porphyrins through regioselective functionalization at the unsubstituted meso position of the macrocycle. Therefore, this approach offers considerable promise for application to the synthesis of trans-A2BC-type porphyrins, including water-soluble derivatives, push-pull chromophores and bis(porphyrin)s.
中文翻译:

A2B 和 A2BC 型卟啉膦酸二酯的通用和可扩展方法
开发了一个两步反应序列,用于从容易获得的反式 A2 型卟啉中获取内消旋-(二烷氧基磷酰基) 卟啉。这种方法涉及溴化和随后的钯催化的膦酰化。在探索了各种反应参数(如溶剂、温度和催化剂)后,确定了这两个步骤的最佳条件。以接近 40% 的总产率制备了一系列二烷氧基磷酰基取代的 A2B-卟啉 Zn3(a-g),其在中间芳基上带有给电子、吸电子或空间大的取代基。这些化合物在空气中稳定,可溶于大多数有机溶剂,是有价值的合成中间体,因为它们可以通过大环未取代的中间位置的区域选择性功能化很容易地转化为功能化的反式 A2BC 型卟啉。因此,这种方法为反式 A2BC 型卟啉的合成提供了广阔的应用前景,包括水溶性衍生物、推挽发色团和双(卟啉)。
更新日期:2016-10-01
中文翻译:

A2B 和 A2BC 型卟啉膦酸二酯的通用和可扩展方法
开发了一个两步反应序列,用于从容易获得的反式 A2 型卟啉中获取内消旋-(二烷氧基磷酰基) 卟啉。这种方法涉及溴化和随后的钯催化的膦酰化。在探索了各种反应参数(如溶剂、温度和催化剂)后,确定了这两个步骤的最佳条件。以接近 40% 的总产率制备了一系列二烷氧基磷酰基取代的 A2B-卟啉 Zn3(a-g),其在中间芳基上带有给电子、吸电子或空间大的取代基。这些化合物在空气中稳定,可溶于大多数有机溶剂,是有价值的合成中间体,因为它们可以通过大环未取代的中间位置的区域选择性功能化很容易地转化为功能化的反式 A2BC 型卟啉。因此,这种方法为反式 A2BC 型卟啉的合成提供了广阔的应用前景,包括水溶性衍生物、推挽发色团和双(卟啉)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号