Chemosphere ( IF 8.1 ) Pub Date : 2022-09-30 , DOI: 10.1016/j.chemosphere.2022.136661 R Manasfi 1 , D Tadić 1 , O Gomez 2 , S Perez 2 , S Chiron 1
|
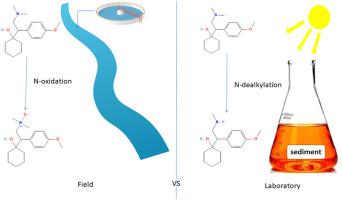
This work aimed at studying the formation and persistence of N-oxides transformation products (TPs) of tertiary amine drugs by combining laboratory and field studies relevant for surface water. A monitoring study using passive samplers was first achieved for assessing attenuation of selected pharmaceuticals and their related N-oxides and N-, O-dealkylated TPs (i.e., venlafaxine, tramadol, amisulpride and sulpiride) along a 1.7 km river stretch between two sampling sites. This study revealed the stability of tramadol-N-oxide, amisulpride-N-oxide and the fast dissipation of O-desmethylvenlafaxine-N-oxide, as well as the significance of N-oxidized TPs in comparison to N-dealkylated TPs and parent compounds in river. Lab-scale experiments were then implemented for a better understanding of their mechanisms of formation and degradation under aerobic water/sediment testing and under simulated solar photochemistry. N-oxidation reactions were always a minor transformation pathway under both degradation conditions with respect to N-and O-dealkylation reactions. The amount of generated N-oxides were similar for venlafaxine, tramadol and sulpiride and peaked in the 8.4–12.8% and <4% of their initial concentration (100 μg/L), during photodegradation and biodegradation experiments, respectively. Other transformation pathways such as hydroxylation and α-C-hydroxylation followed by oxidation to amide or dehydration were also identified. Investigated N-oxides TPs (except O-desmethylvenlafaxine-N-oxide) were found stable under solar photolysis and aerobic biodegradation with a very slight reverse reaction to parent compound observed for tramadol-N-oxide and amisulpride-N-oxide. Lab-scale degradation experiments were not able to anticipate the high occurrence levels of N-oxide compounds in the environment. This was most likely due to faster degradation kinetics and/or higher sorption to sediment of parent compounds and dealkylated TPs over N-oxide TPs, resulting in higher relative accumulation of the latter.
中文翻译:

在实验室和现场研究中叔胺药物的 N-氧化物转化产物的持久性。
这项工作旨在通过结合与地表水相关的实验室和现场研究,研究叔胺类药物的 N-氧化物转化产物 (TPs) 的形成和持久性。首次使用被动采样器进行监测研究,以评估选定药物及其相关 N-氧化物和 N-、O-脱烷基 TP(即文拉法辛、曲马多、氨磺必利和舒必利)沿两个采样点之间的 1.7 公里河段的衰减. 本研究揭示了曲马多-N-氧化物、氨磺必利-N-氧化物的稳定性和 O-去甲基文拉法辛-N-氧化物的快速消散,以及 N-氧化 TPs 与 N-脱烷基化 TPs 和母体化合物相比的重要性在河里。然后实施实验室规模的实验,以更好地了解它们在需氧水/沉积物测试和模拟太阳光化学下的形成和降解机制。在 N-和 O-脱烷基化反应的两种降解条件下,N-氧化反应始终是次要的转化途径。在光降解和生物降解实验中,文拉法辛、曲马多和舒必利的 N-氧化物生成量相似,峰值分别为初始浓度 (100 μg/L) 的 8.4-12.8% 和 <4%。还确定了其他转化途径,例如羟基化和α-C-羟基化,然后氧化成酰胺或脱水。研究的 N-氧化物 TPs(O-去甲基文拉法辛-N-氧化物除外)在太阳光解和有氧生物降解下是稳定的,对曲马多-N-氧化物和氨磺必利-N-氧化物观察到的母体化合物有非常轻微的逆反应。实验室规模的降解实验无法预测环境中 N-氧化物化合物的高发生率。这很可能是由于更快的降解动力学和/或更高的母体化合物和脱烷基化 TPs 对 N-氧化物 TPs 沉积物的吸附,导致后者的相对积累更高。






























 京公网安备 11010802027423号
京公网安备 11010802027423号