当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Methyl Sorbate Catalyzed by Deep Eutectic Solvent Based on Choline Chloride: Kinetics and Optimization
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-09-29 , DOI: 10.1021/acs.iecr.2c02748 Yu Qi 1 , Jumei Xu 1 , Zuoxiang Zeng 1 , Weilan Xue 1 , Zhu Zhu 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-09-29 , DOI: 10.1021/acs.iecr.2c02748 Yu Qi 1 , Jumei Xu 1 , Zuoxiang Zeng 1 , Weilan Xue 1 , Zhu Zhu 1
Affiliation
|
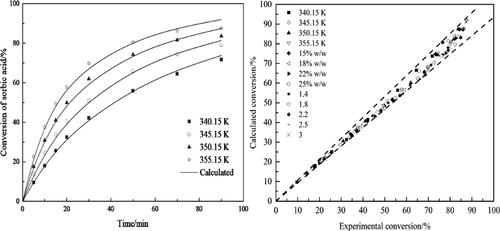
The synthesis process and kinetics of the esterification of methanol with sorbic acid catalyzed by deep eutectic solvents (DESs) were explored in this study. A series of green DESs composed of choline chloride (ChCl) and p-toluene sulfonic acid monohydrate (PTSA) were successfully prepared. Design and optimization of the process were conducted using the response surface methodology with Box-Behnen design. The influences of the value of z (z is the molar ratio of PTSA to ChCl), catalyst loading, methanol/sorbic acid molar ratio, and temperature on the conversion of sorbic acid were evaluated. Using ChCl-1.38PTSA as a catalyst, the kinetic data and chemical equilibrium compositions of the esterification were measured at a temperature range of 340.15–355.15 K. The UNIFAC model was utilized to estimate the equilibrium constants, and the thermodynamic data (ΔrH0, ΔrS0, ΔrG0) of the esterification reaction were calculated as well. The pseudohomogeneous model based on activity was then adopted to describe the reaction kinetics and the model fitted well with the experimental data. The activation energy of the forward and reverse reaction were calculated. In addition, the activity of ChCl-1.38PTSA declined not obviously after five cycles, suggesting that ChCl-1.38PTSA has good stability and recyclability.
中文翻译:

基于氯化胆碱的深共晶溶剂催化山梨酸甲酯的合成:动力学与优化
本研究探讨了深共熔溶剂(DESs)催化甲醇与山梨酸酯化的合成过程和动力学。成功制备了由氯化胆碱(ChCl)和对甲苯磺酸一水合物(PTSA)组成的一系列绿色DES。使用带有 Box-Behnen 设计的响应面方法对过程进行设计和优化。z值的影响(z是 PTSA 与 ChCl) 的摩尔比)、催化剂负载量、甲醇/山梨酸摩尔比和温度对山梨酸转化率的影响。使用 ChCl-1.38PTSA 作为催化剂,在 340.15-355.15 K 的温度范围内测量酯化的动力学数据和化学平衡组成。利用 UNIFAC 模型估计平衡常数,热力学数据(Δr H 0 , Δ r S 0 , Δ r G 0) 的酯化反应也被计算出来。然后采用基于活性的拟均相模型来描述反应动力学,该模型与实验数据拟合良好。计算正向和反向反应的活化能。此外,ChCl-1.38PTSA的活性在5个循环后没有明显下降,说明ChCl-1.38PTSA具有良好的稳定性和可回收性。
更新日期:2022-09-29
中文翻译:

基于氯化胆碱的深共晶溶剂催化山梨酸甲酯的合成:动力学与优化
本研究探讨了深共熔溶剂(DESs)催化甲醇与山梨酸酯化的合成过程和动力学。成功制备了由氯化胆碱(ChCl)和对甲苯磺酸一水合物(PTSA)组成的一系列绿色DES。使用带有 Box-Behnen 设计的响应面方法对过程进行设计和优化。z值的影响(z是 PTSA 与 ChCl) 的摩尔比)、催化剂负载量、甲醇/山梨酸摩尔比和温度对山梨酸转化率的影响。使用 ChCl-1.38PTSA 作为催化剂,在 340.15-355.15 K 的温度范围内测量酯化的动力学数据和化学平衡组成。利用 UNIFAC 模型估计平衡常数,热力学数据(Δr H 0 , Δ r S 0 , Δ r G 0) 的酯化反应也被计算出来。然后采用基于活性的拟均相模型来描述反应动力学,该模型与实验数据拟合良好。计算正向和反向反应的活化能。此外,ChCl-1.38PTSA的活性在5个循环后没有明显下降,说明ChCl-1.38PTSA具有良好的稳定性和可回收性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号