当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halogenated Zn2+ Solvation Structure for Reversible Zn Metal Batteries
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-28 , DOI: 10.1021/jacs.2c06927
Qiu Zhang 1 , Yilin Ma 1 , Yong Lu 1 , Youxuan Ni 1 , Liu Lin 1 , Zhenkun Hao 1 , Zhenhua Yan 1 , Qing Zhao 1 , Jun Chen 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-28 , DOI: 10.1021/jacs.2c06927
Qiu Zhang 1 , Yilin Ma 1 , Yong Lu 1 , Youxuan Ni 1 , Liu Lin 1 , Zhenkun Hao 1 , Zhenhua Yan 1 , Qing Zhao 1 , Jun Chen 1
Affiliation
|
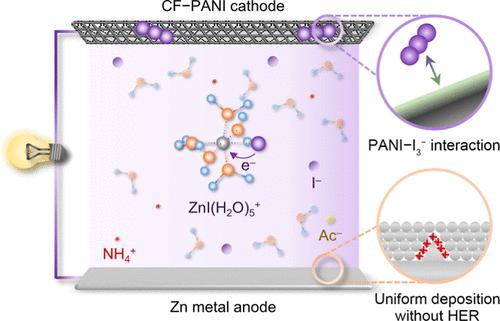
Rechargeable aqueous Zn metal batteries have become promising candidates for large-scale electrochemical energy storage owing to their high safety and affordable low cost. However, Zn metal anode suffers from dendritic growth and hydrogen evolution reaction (HER), deteriorating the electrochemical performance. Here, we demonstrate that these challenges can be conquered by introducing a halogen ion into the Zn2+ solvation structure. By designing an electrolyte composed of zinc acetate and ammonium halide, the electron-donating anion I– can coordinate with Zn2+ and transform the traditional Zn(H2O)62+ to ZnI(H2O)5+, in which I– could transfer electrons into H2O and thus suppress HER. The dynamic electrostatic shielding layer formed by concomitant NH4+ can restrict the dendritic growth. As a result, the halogenated electrolyte achieves a high initial coulombic efficiency (CE) of 99.3% in the Zn plating/stripping process and remains at an average of ∼99.8% with uniform Zn deposition. Moreover, Zn–I batteries are constructed by using dissociative I– as the cathode and carbon felt–polyaniline as the conductive and adsorptive layer, exhibiting an average CE of 98.6% without capacity decay after 300 cycles. This work provides insights into the halogenated Zn2+ solvation structure and offers a general electrolyte design strategy for achieving a highly reversible Zn metal anode and batteries.
中文翻译:

用于可逆锌金属电池的卤化 Zn2+ 溶剂化结构
可充电水系锌金属电池因其高安全性和可负担的低成本而成为大规模电化学储能的有希望的候选者。然而,锌金属负极会发生枝晶生长和析氢反应(HER),从而降低电化学性能。在这里,我们证明可以通过将卤素离子引入 Zn 2+溶剂化结构来克服这些挑战。通过设计由乙酸锌和卤化铵组成的电解液,给电子阴离子 I -可以与 Zn 2+配位,将传统的 Zn(H 2 O) 6 2+转化为 ZnI(H 2 O) 5 +,其中我–可以将电子转移到 H 2 O 中,从而抑制 HER。伴随NH 4 +形成的动态静电屏蔽层可以限制枝晶的生长。结果,卤化电解液在锌电镀/剥离过程中达到了 99.3% 的高初始库仑效率 (CE),并且在均匀的锌沉积下保持在 99.8% 的平均水平。此外,Zn - I 电池采用离解 I作为正极,碳毡聚苯胺作为导电和吸附层,在 300 次循环后平均 CE 为 98.6%,容量没有衰减。这项工作提供了对卤化 Zn 2+的深入了解溶剂化结构,并为实现高度可逆的锌金属负极和电池提供了一种通用的电解质设计策略。
更新日期:2022-09-28
中文翻译:

用于可逆锌金属电池的卤化 Zn2+ 溶剂化结构
可充电水系锌金属电池因其高安全性和可负担的低成本而成为大规模电化学储能的有希望的候选者。然而,锌金属负极会发生枝晶生长和析氢反应(HER),从而降低电化学性能。在这里,我们证明可以通过将卤素离子引入 Zn 2+溶剂化结构来克服这些挑战。通过设计由乙酸锌和卤化铵组成的电解液,给电子阴离子 I -可以与 Zn 2+配位,将传统的 Zn(H 2 O) 6 2+转化为 ZnI(H 2 O) 5 +,其中我–可以将电子转移到 H 2 O 中,从而抑制 HER。伴随NH 4 +形成的动态静电屏蔽层可以限制枝晶的生长。结果,卤化电解液在锌电镀/剥离过程中达到了 99.3% 的高初始库仑效率 (CE),并且在均匀的锌沉积下保持在 99.8% 的平均水平。此外,Zn - I 电池采用离解 I作为正极,碳毡聚苯胺作为导电和吸附层,在 300 次循环后平均 CE 为 98.6%,容量没有衰减。这项工作提供了对卤化 Zn 2+的深入了解溶剂化结构,并为实现高度可逆的锌金属负极和电池提供了一种通用的电解质设计策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号