Science China Materials ( IF 6.8 ) Pub Date : 2022-09-23 , DOI: 10.1007/s40843-022-2179-7 Zhenhua Liu , Yanpeng Liu , Yanan Zhang , Xiaoli Liu , De Yan , Juanjuan Huang , Shanglong Peng
|
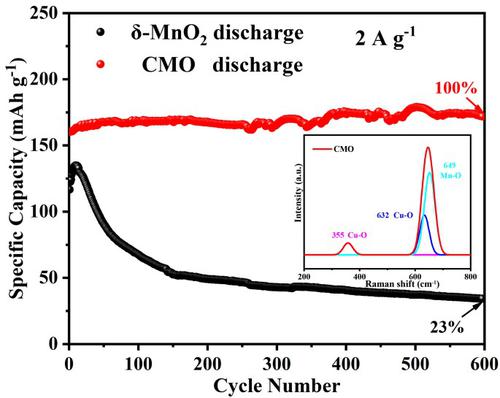
Ion intercalation is an effective strategy for improving the cycle stability and rate performance of δ-MnO2 as a cathode material for aqueous zinc-ion batteries. However, in practice, ion selection appears rather arbitrary. In this work, Cu2+ was chosen for δ-MnO2 intercalation because although Cu2+ and Zn2+ have similar diameters, Cu2+ has a slightly higher electronegativity (1.359) than Zn2+ (1.347). Therefore, Cu2+ has a stronger interaction with the MnO2 lattice than Zn2+ and can be stable during the intercalation/deintercalation of Zn2+ and H+. Results showed that the performance of Cu-doped δ-MnO2 (CMO) was greatly improved. Moreover, at the high current density of 2 A g−1, CMO achieved excellent cycle stability with 100% capacity retention after 600 cycles, whereas pristine δ-MnO2 exhibited only 23% capacity retention. When the current density was increased from 0.2 to 2.0 A g−1, the CMO electrode also delivered remarkable rate performance with 72% capacity retention, which was considerably higher than the 32% capacity retention demonstrated by pristine δ-MnO2. Given that Cu2+ has a greater electronegativity than Zn2+, the Cu-O bond formed in CMO acted as a stable structural column and greatly improved the stability of CMO. Cu2+ doping also increased the electronic conductivity and ionic conductivity of CMO and reduced the charge transfer resistance of H+ and Zn2+ at the electrode/electrolyte interface, which improved the rate performance of CMO greatly. This work provides new insights into intercalation strategies to improve the electrochemical performance of batteries.
中文翻译:

从电负性角度选择Cu2+插层:提高水系锌离子电池δ-MnO2正极材料的循环稳定性和倍率性能
离子嵌入是提高δ-MnO 2作为水系锌离子电池正极材料循环稳定性和倍率性能的有效策略。然而,在实践中,离子选择显得相当随意。在这项工作中,选择 Cu 2+用于 δ-MnO 2插层,因为尽管 Cu 2+和 Zn 2+具有相似的直径,但 Cu 2+的电负性 (1.359) 略高于 Zn 2+ (1.347)。因此,Cu 2+与MnO 2晶格的相互作用比Zn 2+强,在Zn 2+和H的嵌入/脱嵌过程中可以保持稳定+。结果表明,Cu掺杂的δ-MnO 2 (CMO)的性能得到了极大的改善。此外,在 2 A g -1的高电流密度下,CMO 在 600 次循环后实现了出色的循环稳定性和 100% 的容量保持率,而原始的 δ-MnO 2仅表现出 23% 的容量保持率。当电流密度从 0.2 增加到 2.0 A g -1时,CMO 电极还提供了显着的倍率性能和 72% 的容量保持率,这远高于原始 δ-MnO 2所展示的 32% 的容量保持率。假设 Cu 2+比 Zn 2+具有更大的电负性,CMO中形成的Cu-O键作为稳定的结构柱,大大提高了CMO的稳定性。Cu 2+掺杂还提高了CMO的电子电导率和离子电导率,降低了H +和Zn 2+在电极/电解质界面的电荷转移电阻,大大提高了CMO的倍率性能。这项工作为提高电池电化学性能的插层策略提供了新的见解。













































 京公网安备 11010802027423号
京公网安备 11010802027423号