当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nature and Dynamic Evolution of Rh Single Atoms Trapped by CeO2 in CO Hydrogenation
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-26 , DOI: 10.1021/acscatal.2c02103 Danfeng Wu 1, 2 , Sixu Liu 1, 2 , Mingqi Zhong 1 , Jiafei Zhao 1 , Congcong Du 1 , Yanling Yang 3 , Yifei Sun 3 , Jingdong Lin 1 , Shaolong Wan 1 , Shuai Wang 1 , Jianyu Huang 4 , Yali Yao 5 , Zhe Li 6 , Haifeng Xiong 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-26 , DOI: 10.1021/acscatal.2c02103 Danfeng Wu 1, 2 , Sixu Liu 1, 2 , Mingqi Zhong 1 , Jiafei Zhao 1 , Congcong Du 1 , Yanling Yang 3 , Yifei Sun 3 , Jingdong Lin 1 , Shaolong Wan 1 , Shuai Wang 1 , Jianyu Huang 4 , Yali Yao 5 , Zhe Li 6 , Haifeng Xiong 1, 2
Affiliation
|
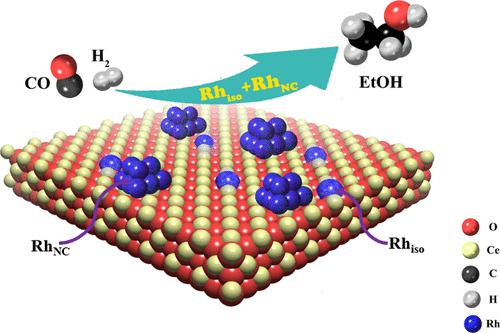
Metal single-atom catalysts (SACs) have attracted extensive interest because of their maximum atom efficiency and potentially good performances. However, the understanding of the catalytic behavior and the stability of SACs in high-temperature catalytic reactions remains a major challenge, especially under reducing conditions. In this work, we investigated the nature and stability of Rh/CeO2 SACs with different Rh loadings (0.2–4 wt % Rh) during syngas conversion (260 °C, 2 MPa). Using CO-DRIFTS, we found that atomically dispersed Rh was dispersed on Rh/CeO2 catalysts with an Rh loading up to 3 wt %. The Rh/CeO2 catalysts were stable in H2 up to 350 °C, while Rh nanoclusters were formed in CO at 200 °C. During syngas conversion at 260 °C, the Rh single atoms (Rhiso) on a 0.5 wt % Rh/CeO2 SAC slowly agglomerated to form Rh nanoclusters (RhNC) and a Rhiso/RhNC ratio of ∼1:2.5 was obtained after ∼10 h time-on-stream run. Furthermore, the Rhiso/RhNC ratio did not change with further reaction, indicating that the reaction reached steady state after a 10 h run. After the catalysts reached steady state (∼10 h), the Rhiso/RhNC ratio on the spent Rh/CeO2 catalysts decreased with the increase in Rh loadings, while the reactivity increased first and then decreased. We observed an optimal value of Rhiso/RhNC ≈ 1:3 on the 1 wt % Rh/CeO2 catalyst, showing the maximum reaction rates of CO (rCO) and ethanol selectivity (SEtOH) of 85.8 μmol/s/gRh and 26.2%, respectively. This indicated that the combination of Rh single atoms and Rh nanoclusters is beneficial to the ethanol selectivity and reaction rate, which was confirmed by DFT simulations. Moreover, the ratios of Rhiso/RhNC on the spent catalysts under the steady state have good relationships with the CO conversion rate and product selectivity in the reaction.
中文翻译:

CO加氢中CeO2捕获的Rh单原子的性质及动态演化
金属单原子催化剂(SACs)因其最大的原子效率和潜在的良好性能而引起了广泛的兴趣。然而,了解高温催化反应中 SAC 的催化行为和稳定性仍然是一项重大挑战,尤其是在还原条件下。在这项工作中,我们研究了在合成气转化(260 °C,2 MPa)期间具有不同 Rh 负载量(0.2-4 wt % Rh)的 Rh/CeO 2 SACs的性质和稳定性。使用 CO-DRIFTS,我们发现原子分散的 Rh 分散在 Rh/CeO 2催化剂上,Rh 负载量高达 3 wt%。Rh/CeO 2催化剂在H 2中是稳定的高达 350 °C,而 Rh 纳米团簇在 200 °C 的 CO 中形成。在 260 °C 的合成气转化过程中,0.5 wt% Rh/CeO 2 SAC 上的 Rh 单原子 (Rh iso )缓慢团聚形成 Rh 纳米团簇 (Rh NC ),Rh iso /Rh NC比约为 1:2.5在 10 小时的在线运行时间后获得。此外,Rh iso /Rh NC比率没有随着进一步反应而改变,表明反应在运行 10 小时后达到稳定状态。催化剂达到稳态后(~10 h),废Rh/CeO 2上的Rh iso /Rh NC比值催化剂随着Rh负载量的增加而降低,而反应活性先升高后降低。我们在 1 wt % Rh/CeO 2催化剂上观察到 Rh iso /Rh NC ≈ 1:3的最佳值,显示 CO ( r CO )的最大反应速率和乙醇选择性 ( S EtOH ) 为 85.8 μmol/s/ g Rh和 26.2%,分别。这表明Rh单原子和Rh纳米团簇的组合有利于乙醇的选择性和反应速率,这通过DFT模拟得到证实。此外,Rh iso /Rh NC的比率在稳态下对废催化剂的影响与反应中的CO转化率和产物选择性有很好的关系。
更新日期:2022-09-26
中文翻译:

CO加氢中CeO2捕获的Rh单原子的性质及动态演化
金属单原子催化剂(SACs)因其最大的原子效率和潜在的良好性能而引起了广泛的兴趣。然而,了解高温催化反应中 SAC 的催化行为和稳定性仍然是一项重大挑战,尤其是在还原条件下。在这项工作中,我们研究了在合成气转化(260 °C,2 MPa)期间具有不同 Rh 负载量(0.2-4 wt % Rh)的 Rh/CeO 2 SACs的性质和稳定性。使用 CO-DRIFTS,我们发现原子分散的 Rh 分散在 Rh/CeO 2催化剂上,Rh 负载量高达 3 wt%。Rh/CeO 2催化剂在H 2中是稳定的高达 350 °C,而 Rh 纳米团簇在 200 °C 的 CO 中形成。在 260 °C 的合成气转化过程中,0.5 wt% Rh/CeO 2 SAC 上的 Rh 单原子 (Rh iso )缓慢团聚形成 Rh 纳米团簇 (Rh NC ),Rh iso /Rh NC比约为 1:2.5在 10 小时的在线运行时间后获得。此外,Rh iso /Rh NC比率没有随着进一步反应而改变,表明反应在运行 10 小时后达到稳定状态。催化剂达到稳态后(~10 h),废Rh/CeO 2上的Rh iso /Rh NC比值催化剂随着Rh负载量的增加而降低,而反应活性先升高后降低。我们在 1 wt % Rh/CeO 2催化剂上观察到 Rh iso /Rh NC ≈ 1:3的最佳值,显示 CO ( r CO )的最大反应速率和乙醇选择性 ( S EtOH ) 为 85.8 μmol/s/ g Rh和 26.2%,分别。这表明Rh单原子和Rh纳米团簇的组合有利于乙醇的选择性和反应速率,这通过DFT模拟得到证实。此外,Rh iso /Rh NC的比率在稳态下对废催化剂的影响与反应中的CO转化率和产物选择性有很好的关系。

































 京公网安备 11010802027423号
京公网安备 11010802027423号