Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2022-09-24 , DOI: 10.1016/j.molliq.2022.120445 Reza Nakhaei-Kohani , Saeid Atashrouz , Fahimeh Hadavimoghaddam , Ali Abedi , Karam Jabbour , Abdolhossein Hemmati-Sarapardeh , Ahmad Mohaddespour
|
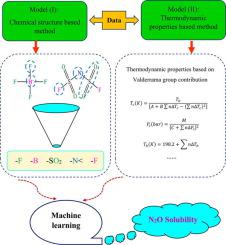
In past decades, nitrous oxide (N2O), a strong greenhouse gas, has become a serious transdisciplinary issue. As a result, removing N2O utilizing strong green solvents like ionic liquids (ILs) has emerged as a popular method of lowering N2O levels in the environment. The ability to accurately estimate N2O solubility in ILs provides a deeper understanding of the ILs' performance as a solvent for the elimination and management of this hazardous gaseous contaminant in the atmosphere. For the implementation of future IL-based separation procedures at massive scales, reliable calculation of this critical factor is required. The purpose of this research was to develop reliable intelligent networks that can estimate N2O solubility in diverse ILs. To this end, four powerful intelligent models including deep belief network (DBN), categorical boosting algorithm (Cat-Boost), extreme learning machine (ELM), and extreme gradient boosting (XGB) were developed based on two distinct methods, (I): Chemical structure-based and (II): Thermodynamic properties-based methods. Also, different equations of state (EOSs) were employed to compare their performance with smart models. The acquired findings indicate that the novel approaches appropriately estimate the solubility of N2O in ILs. Furthermore, the XGB approach was discovered to be the superior forecasting technique in both methods ((I): R2 = 0.9999 and RMSE = 0.0016, (II): R2 = 0.9998 and RMSE = 0.0025). The sensitivity analysis of the XGB models revealed that pressure has the greatest effect on the solubility values in both strategies with an absolute relevancy factor value of 0.74, and concerning the chemical structures of ionic liquids, the –SO2 substructure has the greatest effect on N2O solubility with an absolute relevancy factor value of 0.4. In addition, significant superiority of machine learning models over EOSs was observed. Finally, the Leverage approach was used to demonstrate the reliability of the novel paradigm, showing over 96 % of data are into the paradigm's applicability range.
中文翻译:

基于化学结构和热力学性质的模型,用于估计一氧化二氮在离子液体中的溶解度:状态方程和机器学习方法
在过去的几十年中,强温室气体一氧化二氮(N 2 O)已成为一个严重的跨学科问题。因此,使用离子液体 (ILs) 等强绿色溶剂去除 N 2 O 已成为降低环境中 N 2 O 水平的流行方法。准确估计离子液体中 N 2 O 溶解度的能力可以更深入地了解离子液体 作为溶剂的性能,以消除和管理大气中的这种有害气体污染物。对于未来大规模实施基于 IL 的分离程序, 需要对该关键因素进行可靠计算。本研究的目的是开发可靠的智能网络,可以估计 N2 O 在不同 IL 中的溶解度。为此,基于两种不同的方法开发了四种强大的智能模型,包括深度信念网络(DBN)、分类提升算法(Cat-Boost)、极限学习机(ELM)和极限梯度提升(XGB),(I) : 基于化学结构和 (II): 基于热力学性质的方法。此外,还采用了不同的状态方程 (EOS) 来比较它们与智能模型的性能。获得的研究结果表明,新方法适当地 估计了 N 2 O 在 ILs 中的溶解度。此外,XGB 方法被发现是两种方法中的优越预测技术((I):R 2 = 0.9999 和 RMSE = 0.0016,(II):R 2 = 0.9998 和 RMSE = 0.0025)。XGB模型的敏感性分析表明,压力对两种策略的溶解度值影响最大,绝对相关因子值为0.74,离子液体的化学结构中,-SO 2子结构对N的影响最大。2 O 溶解度,绝对相关因子值为 0.4。此外,观察到机器学习模型相对于 EOS 的显着优势。最后,利用杠杆方法证明了新范式的可靠性,表明超过 96% 的数据在范式的适用范围内。

































 京公网安备 11010802027423号
京公网安备 11010802027423号