当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfite-Catalyzed Nucleophilic Substitution Reactions with Thiamin and Analogous Pyrimidine Donors Proceed via an SNAE Mechanism
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-09-23 , DOI: 10.1021/acs.joc.2c01685 Graeme W Howe 1 , Neil L Grenade 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-09-23 , DOI: 10.1021/acs.joc.2c01685 Graeme W Howe 1 , Neil L Grenade 1
Affiliation
|
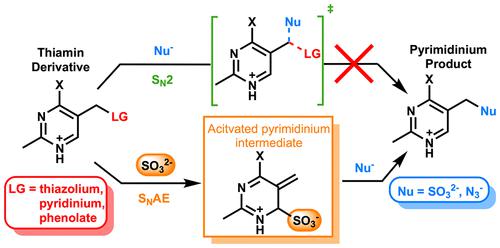
When treated with SO32–, thiamin undergoes a substitution reaction to release a thiazole leaving group and the corresponding sulfonate. Although this reaction could proceed via a simple SN2-like mechanism, a multistep addition–elimination (SNAE) mechanism involving the addition of SO32– to C6′ of the 4-aminopyrimidine of thiamin has also been proposed. Although this reaction has potential utility in the synthesis of substituted pyrimidines and provides a direct analogue to reactions catalyzed by thiaminases, a detailed mechanistic picture of the SO32–-catalyzed cleavage of thiamin has remained elusive. Here, DFT calculations have been used to probe the relative energetics and the factors that shape the potential energy surfaces that define the possible mechanisms of substitution. These calculations provide clear support for the SNAE mechanism over an SN2-like process and illustrate that the unique ability of SO32– to activate thiamin toward nucleophilic displacement is due to the combined nucleophilicity and relatively poor leaving group ability of SO32–. Both of these factors favor the forward partitioning of the sulfite adduct toward the cleavage products whereas adducts formed with other nucleophiles overwhelmingly revert to reactants. Calculations performed with a range of substrates with various electrophilicities and nucleofugalities consistently suggest that the SNAE pathway is significantly lower in energy than the direct substitution, illustrating that this SO32–-catalyzed multistep process is likely to be broadly applicable both in solution and in catalysis by thiaminases.
中文翻译:

硫胺和类似嘧啶供体的亚硫酸盐催化亲核取代反应通过 SNAE 机制进行
当用 SO 3 2-处理时,硫胺素会发生取代反应,释放出噻唑离去基团和相应的磺酸盐。尽管该反应可以通过简单的类似 S N 2 的机制进行,但也有人提出了一种多步加成 - 消除 (S N AE) 机制,涉及将 SO 3 2-添加到硫胺素的 4-氨基嘧啶的 C6' 上。尽管该反应在取代嘧啶的合成中具有潜在用途,并提供了硫胺酶催化反应的直接类似物,但 SO 3 2-的详细机理图硫胺的催化裂解仍然难以捉摸。在这里,DFT 计算已被用于探测相对能量学和形成定义可能替代机制的势能表面的因素。这些计算为类 S N 2 过程的 S N AE 机制提供了明确的支持,并说明 SO 3 2-激活硫胺素向亲核置换的独特能力是由于 SO 的亲核性和相对较差的离去基团能力的结合。3 2–. 这两个因素都有利于亚硫酸盐加合物向裂解产物的前向分配,而与其他亲核试剂形成的加合物压倒性地恢复为反应物。对一系列具有各种亲电性和离核性的底物进行的计算一致表明,S N AE 途径的能量明显低于直接取代,说明这种 SO 3 2-催化的多步过程可能在溶液中都广泛适用在硫胺酶的催化下。
更新日期:2022-09-23
中文翻译:

硫胺和类似嘧啶供体的亚硫酸盐催化亲核取代反应通过 SNAE 机制进行
当用 SO 3 2-处理时,硫胺素会发生取代反应,释放出噻唑离去基团和相应的磺酸盐。尽管该反应可以通过简单的类似 S N 2 的机制进行,但也有人提出了一种多步加成 - 消除 (S N AE) 机制,涉及将 SO 3 2-添加到硫胺素的 4-氨基嘧啶的 C6' 上。尽管该反应在取代嘧啶的合成中具有潜在用途,并提供了硫胺酶催化反应的直接类似物,但 SO 3 2-的详细机理图硫胺的催化裂解仍然难以捉摸。在这里,DFT 计算已被用于探测相对能量学和形成定义可能替代机制的势能表面的因素。这些计算为类 S N 2 过程的 S N AE 机制提供了明确的支持,并说明 SO 3 2-激活硫胺素向亲核置换的独特能力是由于 SO 的亲核性和相对较差的离去基团能力的结合。3 2–. 这两个因素都有利于亚硫酸盐加合物向裂解产物的前向分配,而与其他亲核试剂形成的加合物压倒性地恢复为反应物。对一系列具有各种亲电性和离核性的底物进行的计算一致表明,S N AE 途径的能量明显低于直接取代,说明这种 SO 3 2-催化的多步过程可能在溶液中都广泛适用在硫胺酶的催化下。































 京公网安备 11010802027423号
京公网安备 11010802027423号