当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Crystal Structures, and Biological Activity Evaluation of Novel Xanthine Derivatives Containing a Pyrethroid Moiety
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-09-22 , DOI: 10.1021/acs.jafc.2c03876 Shuyun Zhang 1 , Lei Wang 1 , Hang Liu 1 , Na Yang 1 , Shujing Yu 1 , Baolei Wang 1
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2022-09-22 , DOI: 10.1021/acs.jafc.2c03876 Shuyun Zhang 1 , Lei Wang 1 , Hang Liu 1 , Na Yang 1 , Shujing Yu 1 , Baolei Wang 1
Affiliation
|
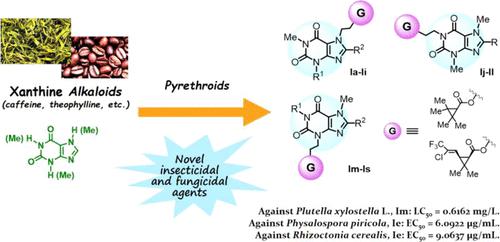
On the basis of the structures of natural xanthines and pyrethroid insecticides, a series of novel xanthine derivatives Ia–Is containing pyrethroid motifs were synthesized and identified by means of melting points, 1H NMR, 13C NMR, and HRMS. The single crystals of compounds In and Iq were obtained, which further confirmed the structures and configurations of this type of compounds. The biological tests showed that some of them exhibited favorable insecticidal activities toward Mythimna separata Walker and Plutella xylostella L. and were superior to the natural methylxanthine compound caffeine and comparable with the insecticide tetramethrin (e.g., compound Im: LC50 = 0.6162 mg/L, against P. xylostella). Among others, Im, Ib, Ij, and Ik could serve as new insecticidal leading structures for further study. Moreover, some of the compounds showed favorable fungicidal activities against a broad spectrum of plant pathogenic fungi (e.g., compound Ie: EC50 = 6.0922 μg/mL, against Physalospora piricola; EC50 = 9.0637 μg/mL, against Rhizoctonia cerealis), which in turn would be an exciting new finding in xanthine chemistry; Ie, Ih, and Ii could be novel fungicidal leading compounds for further investigation. The structure–activity relationships of the compounds were also analyzed and discussed in detail. The research results presented in this paper provide a useful reference and guidance for the development of new natural product-based agrochemicals.
中文翻译:

含有拟除虫菊酯部分的新型黄嘌呤衍生物的合成、晶体结构和生物活性评价
在天然黄嘌呤类和拟除虫菊酯类杀虫剂结构的基础上,合成了一系列含有拟除虫菊酯基序的新型黄嘌呤衍生物Ia-Is,并通过熔点、1 H NMR、13 C NMR 和 HRMS 鉴定。得到了化合物In和Iq的单晶,进一步证实了该类化合物的结构和构型。生物学试验表明,其中一些对Mythimna separata Walker和Plutella xylostella L.表现出良好的杀虫活性,优于天然甲基黄嘌呤化合物咖啡因,与杀虫剂菊酯(如复方Im:LC 50 = 0.6162 mg/L,针对小菜蛾)。其中,Im、Ib、Ij和Ik可以作为新的杀虫主导结构进行进一步研究。此外,一些化合物对广谱植物病原真菌表现出良好的杀真菌活性(例如,化合物Ie:EC 50 = 6.0922 μg/mL,对Physalospora piricola;EC 50 = 9.0637 μg/mL,对谷丝核菌),这反过来,这将是黄嘌呤化学中令人兴奋的新发现;即, Ih和Ii可能是用于进一步研究的新型杀真菌先导化合物。还详细分析和讨论了化合物的构效关系。本文的研究成果为新型天然产物农用化学品的开发提供了有益的参考和指导。
更新日期:2022-09-22
中文翻译:

含有拟除虫菊酯部分的新型黄嘌呤衍生物的合成、晶体结构和生物活性评价
在天然黄嘌呤类和拟除虫菊酯类杀虫剂结构的基础上,合成了一系列含有拟除虫菊酯基序的新型黄嘌呤衍生物Ia-Is,并通过熔点、1 H NMR、13 C NMR 和 HRMS 鉴定。得到了化合物In和Iq的单晶,进一步证实了该类化合物的结构和构型。生物学试验表明,其中一些对Mythimna separata Walker和Plutella xylostella L.表现出良好的杀虫活性,优于天然甲基黄嘌呤化合物咖啡因,与杀虫剂菊酯(如复方Im:LC 50 = 0.6162 mg/L,针对小菜蛾)。其中,Im、Ib、Ij和Ik可以作为新的杀虫主导结构进行进一步研究。此外,一些化合物对广谱植物病原真菌表现出良好的杀真菌活性(例如,化合物Ie:EC 50 = 6.0922 μg/mL,对Physalospora piricola;EC 50 = 9.0637 μg/mL,对谷丝核菌),这反过来,这将是黄嘌呤化学中令人兴奋的新发现;即, Ih和Ii可能是用于进一步研究的新型杀真菌先导化合物。还详细分析和讨论了化合物的构效关系。本文的研究成果为新型天然产物农用化学品的开发提供了有益的参考和指导。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号