当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Manipulating the d-band centers of transition metal phosphides through dual metal doping towards robust overall water splitting
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-09-22 , DOI: 10.1039/d2ta04951a Yunqing Liu 1 , Zuhao Zhang 1 , Lu Zhang 1 , Yuguo Xia 2 , Haiqing Wang 1 , Hong Liu 1, 3 , Shenguang Ge 1 , Jinghua Yu 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-09-22 , DOI: 10.1039/d2ta04951a Yunqing Liu 1 , Zuhao Zhang 1 , Lu Zhang 1 , Yuguo Xia 2 , Haiqing Wang 1 , Hong Liu 1, 3 , Shenguang Ge 1 , Jinghua Yu 4
Affiliation
|
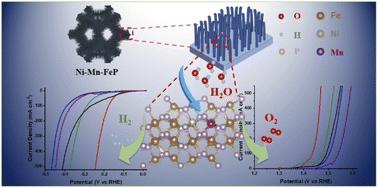
Integrating bifunctional electrocatalysts into one electrolytic cell for water electrolysis has exhibited great convenience and high efficiency in green hydrogen production. However, the opportune adsorptions of intermediate oxygen and hydrogen species on catalytic active sites are prerequisites for the design of high-performing bifunctional electrocatalysts to simultaneously drive oxygen and hydrogen evolution reactions (OER/HER). In this work, we developed a dual metal doping strategy to manipulate the d-band centers of non-precious transition metal phosphides aiming at optimal intermediate adsorption towards robust overall water splitting. The Ni and Mn atom incorporated FeP nanoarrays (Ni–Mn–FeP) were grown directly on NiFe foam via sequential etching-depositing and phosphorization processes. As for the well-known sluggish OER process, Ni–Mn–FeP only requires an overpotential of 185 mV to deliver 10 mA cm−2 in alkaline media. Meanwhile, the HER can also be driven at a low overpotential of 103 mV. In particular, when bifunctional Ni–Mn–FeP as both the cathode and anode is assembled into an electrolytic cell, the electrolysis current of 100 mA cm−2 can be easily achieved at a low cell voltage of 1.55 V and the stability at 500 mA cm−2 can last for 360 h, implying great prospects for large-scale applications. The d-band center theory indicated that the intrinsic high electroactivity of bifunctional Ni–Mn–FeP should arise from the doping induced notable promotions in *O to *OOH conversion and H* adsorption processes. The collaboration approach of codoped low-valence and high-valence metals may inspire the development of high-performance and versatile catalysts.
中文翻译:

通过双金属掺杂操纵过渡金属磷化物的 d 带中心以实现稳健的整体水分解
将双功能电催化剂集成到一个电解槽中用于水电解,在绿色制氢方面表现出极大的便利性和高效性。然而,中间体氧和氢物质在催化活性位点上的适时吸附是设计高性能双功能电催化剂以同时驱动析氧和析氢反应 (OER/HER) 的先决条件。在这项工作中,我们开发了一种双金属掺杂策略来操纵非贵重过渡金属磷化物的 d 带中心,旨在实现稳健的整体水分解的最佳中间吸附。Ni和Mn原子结合的FeP纳米阵列(Ni-Mn-FeP)通过直接在NiFe泡沫上生长顺序蚀刻沉积和磷化工艺。至于众所周知的缓慢 OER 工艺,Ni-Mn-FeP 仅需要 185 mV 的过电位即可在碱性介质中提供 10 mA cm -2的电流。同时,HER 也可以在 103 mV 的低过电位下驱动。特别是,当将双功能Ni-Mn-FeP作为阴极和阳极组装到电解池中时,在1.55 V的低电池电压和500 mA的稳定性下,可以很容易地实现100 mA cm -2的电解电流。厘米-2可持续360小时,大规模应用前景广阔。d 带中心理论表明,双功能 Ni-Mn-FeP 的固有高电活性应该源于掺杂在 *O 到 *OOH 转化和 H* 吸附过程中的显着促进作用。共掺杂低价和高价金属的合作方法可能会激发高性能和多功能催化剂的开发。
更新日期:2022-09-22
中文翻译:

通过双金属掺杂操纵过渡金属磷化物的 d 带中心以实现稳健的整体水分解
将双功能电催化剂集成到一个电解槽中用于水电解,在绿色制氢方面表现出极大的便利性和高效性。然而,中间体氧和氢物质在催化活性位点上的适时吸附是设计高性能双功能电催化剂以同时驱动析氧和析氢反应 (OER/HER) 的先决条件。在这项工作中,我们开发了一种双金属掺杂策略来操纵非贵重过渡金属磷化物的 d 带中心,旨在实现稳健的整体水分解的最佳中间吸附。Ni和Mn原子结合的FeP纳米阵列(Ni-Mn-FeP)通过直接在NiFe泡沫上生长顺序蚀刻沉积和磷化工艺。至于众所周知的缓慢 OER 工艺,Ni-Mn-FeP 仅需要 185 mV 的过电位即可在碱性介质中提供 10 mA cm -2的电流。同时,HER 也可以在 103 mV 的低过电位下驱动。特别是,当将双功能Ni-Mn-FeP作为阴极和阳极组装到电解池中时,在1.55 V的低电池电压和500 mA的稳定性下,可以很容易地实现100 mA cm -2的电解电流。厘米-2可持续360小时,大规模应用前景广阔。d 带中心理论表明,双功能 Ni-Mn-FeP 的固有高电活性应该源于掺杂在 *O 到 *OOH 转化和 H* 吸附过程中的显着促进作用。共掺杂低价和高价金属的合作方法可能会激发高性能和多功能催化剂的开发。

































 京公网安备 11010802027423号
京公网安备 11010802027423号