当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conversion of Aryl Azides to Aminopyridines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-22 , DOI: 10.1021/jacs.2c08464 Sajan C Patel 1 , Noah Z Burns 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-22 , DOI: 10.1021/jacs.2c08464 Sajan C Patel 1 , Noah Z Burns 1
Affiliation
|
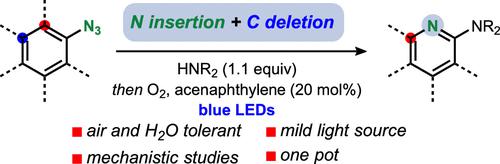
A longstanding challenge in fundamental functional group interconversion has been the direct transformation of benzene into pyridine via nitrogen insertion and carbon deletion. Herein, we report a protocol for the transformation of aryl azides, easily accessible from their corresponding anilines, to 2-aminopyridines using blue light and oxygen. Mechanistic studies corroborate that the arene to pyridine conversion is achieved by nitrogen insertion into the benzene ring followed by oxidative carbon extrusion.
中文翻译:

芳基叠氮化物转化为氨基吡啶
基本官能团相互转化的长期挑战是通过氮插入和碳缺失将苯直接转化为吡啶。在这里,我们报告了一种使用蓝光和氧气将芳基叠氮化物转化为 2-氨基吡啶的方案,该方案很容易从相应的苯胺中获得。机理研究证实,芳烃向吡啶的转化是通过将氮插入苯环,然后进行氧化碳挤出来实现的。
更新日期:2022-09-22
中文翻译:

芳基叠氮化物转化为氨基吡啶
基本官能团相互转化的长期挑战是通过氮插入和碳缺失将苯直接转化为吡啶。在这里,我们报告了一种使用蓝光和氧气将芳基叠氮化物转化为 2-氨基吡啶的方案,该方案很容易从相应的苯胺中获得。机理研究证实,芳烃向吡啶的转化是通过将氮插入苯环,然后进行氧化碳挤出来实现的。































 京公网安备 11010802027423号
京公网安备 11010802027423号