当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Access to Thiocyano-Thioesters from Cyclic Thioacetals via Photoredox Catalysis: An Introduction of Two Functional Groups in One Pot
Organic Letters ( IF 4.9 ) Pub Date : 2022-09-19 , DOI: 10.1021/acs.orglett.2c02601 Pankaj D Dharpure 1 , Mousumi Behera 1 , Vikas V Khade 1 , Archana S Thube 1 , Ramakrishna G Bhat 1
Organic Letters ( IF 4.9 ) Pub Date : 2022-09-19 , DOI: 10.1021/acs.orglett.2c02601 Pankaj D Dharpure 1 , Mousumi Behera 1 , Vikas V Khade 1 , Archana S Thube 1 , Ramakrishna G Bhat 1
Affiliation
|
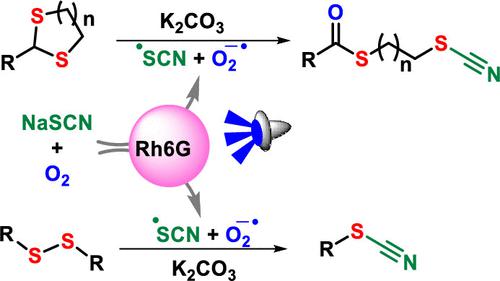
The cyanation of organic compounds is an important synthetic transformation and mainly relies on a toxic CN source. Undeniably, thiocyanate salt has emerged as a very mild and environmentally benign CN source, yet its synthetic utility for cyanation is highly limited to very few types of organic compounds. Herein, we report the direct cyanation of cyclic thioacetals for accessing compounds with two different functional groups (thiocyano-thioesters) in one pot using sodium thiocyanate via photoredox catalysis. The protocol has been further extended for the direct cyanation of disulfides and diselenide to access aryl thiocyanates and aryl selenocyanate. A plausible mechanism has been proposed based on a series of control experiments, cyclic voltammetry and Stern–Volmer studies.
中文翻译:

通过光氧化还原催化从环状硫缩醛中直接获得硫氰基硫酯:一锅法引入两个官能团
有机化合物的氰化是一种重要的合成转化,主要依赖于有毒的 CN 源。不可否认,硫氰酸盐已成为一种非常温和且对环境无害的 CN 来源,但其用于氰化的合成用途仅限于极少数类型的有机化合物。在此,我们报告了使用硫氰酸钠通过光氧化还原催化在一锅中直接氰化环状硫缩醛,从而获得具有两种不同官能团的化合物(硫氰基硫酯)。该协议已进一步扩展为二硫化物和二硒化物的直接氰化以获取芳基硫氰酸酯和芳基硒氰酸酯。基于一系列控制实验、循环伏安法和 Stern-Volmer 研究,提出了一种似是而非的机制。
更新日期:2022-09-19
中文翻译:

通过光氧化还原催化从环状硫缩醛中直接获得硫氰基硫酯:一锅法引入两个官能团
有机化合物的氰化是一种重要的合成转化,主要依赖于有毒的 CN 源。不可否认,硫氰酸盐已成为一种非常温和且对环境无害的 CN 来源,但其用于氰化的合成用途仅限于极少数类型的有机化合物。在此,我们报告了使用硫氰酸钠通过光氧化还原催化在一锅中直接氰化环状硫缩醛,从而获得具有两种不同官能团的化合物(硫氰基硫酯)。该协议已进一步扩展为二硫化物和二硒化物的直接氰化以获取芳基硫氰酸酯和芳基硒氰酸酯。基于一系列控制实验、循环伏安法和 Stern-Volmer 研究,提出了一种似是而非的机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号