Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Behavior of Citrate-Capped Ultrasmall Gold Nanoparticles on a Supported Lipid Bilayer Interface at Atomic Resolution
ACS Nano ( IF 15.8 ) Pub Date : 2022-09-19 , DOI: 10.1021/acsnano.2c07751 Rashad Kariuki 1 , Rowan Penman 1 , Saffron J Bryant 1 , Rebecca Orrell-Trigg 1 , Nastaran Meftahi 2 , Russell J Crawford 1 , Chris F McConville 1, 3 , Gary Bryant 1 , Kislon Voïtchovsky 4 , Charlotte E Conn 1 , Andrew J Christofferson 1, 2 , Aaron Elbourne 1
ACS Nano ( IF 15.8 ) Pub Date : 2022-09-19 , DOI: 10.1021/acsnano.2c07751 Rashad Kariuki 1 , Rowan Penman 1 , Saffron J Bryant 1 , Rebecca Orrell-Trigg 1 , Nastaran Meftahi 2 , Russell J Crawford 1 , Chris F McConville 1, 3 , Gary Bryant 1 , Kislon Voïtchovsky 4 , Charlotte E Conn 1 , Andrew J Christofferson 1, 2 , Aaron Elbourne 1
Affiliation
|
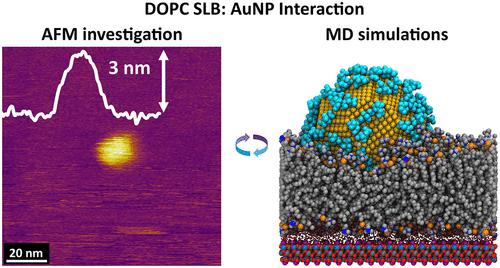
Nanomaterials have the potential to transform biological and biomedical research, with applications ranging from drug delivery and diagnostics to targeted interference of specific biological processes. Most existing research is aimed at developing nanomaterials for specific tasks such as enhanced biocellular internalization. However, fundamental aspects of the interactions between nanomaterials and biological systems, in particular, membranes, remain poorly understood. In this study, we provide detailed insights into the molecular mechanisms governing the interaction and evolution of one of the most common synthetic nanomaterials in contact with model phospholipid membranes. Using a combination of atomic force microscopy (AFM) and molecular dynamics (MD) simulations, we elucidate the precise mechanisms by which citrate-capped 5 nm gold nanoparticles (AuNPs) interact with supported lipid bilayers (SLBs) of pure fluid (DOPC) and pure gel-phase (DPPC) phospholipids. On fluid-phase DOPC membranes, the AuNPs adsorb and are progressively internalized as the citrate capping of the NPs is displaced by the surrounding lipids. AuNPs also interact with gel-phase DPPC membranes where they partially embed into the outer leaflet, locally disturbing the lipid organization. In both systems, the AuNPs cause holistic perturbations throughout the bilayers. AFM shows that the lateral diffusion of the particles is several orders of magnitude smaller than that of the lipid molecules, which creates some temporary scarring of the membrane surface. Our results reveal how functionalized AuNPs interact with differing biological membranes with mechanisms that could also have implications for cooperative membrane effects with other molecules.
中文翻译:

柠檬酸盐封端的超小金纳米粒子在支持的脂质双层界面上的原子分辨率行为
纳米材料具有改变生物和生物医学研究的潜力,其应用范围从药物输送和诊断到特定生物过程的靶向干扰。大多数现有研究旨在开发用于特定任务的纳米材料,例如增强生物细胞内化。然而,纳米材料与生物系统,特别是膜之间相互作用的基本方面仍然知之甚少。在这项研究中,我们对控制与模型磷脂膜接触的最常见合成纳米材料之一的相互作用和进化的分子机制提供了详细的见解。结合使用原子力显微镜 (AFM) 和分子动力学 (MD) 模拟,我们阐明了柠檬酸盐封端的 5 nm 金纳米粒子 (AuNP) 与纯流体 (DOPC) 和纯凝胶相 (DPPC) 磷脂的支持脂质双层 (SLB) 相互作用的精确机制。在液相 DOPC 膜上,随着 NPs 的柠檬酸盐帽被周围的脂质置换,AuNPs 吸附并逐渐内化。AuNPs 还与凝胶相 DPPC 膜相互作用,它们部分嵌入外叶,局部干扰脂质组织。在这两个系统中,AuNP 都会引起整个双层的整体扰动。AFM 显示颗粒的横向扩散比脂质分子的横向扩散小几个数量级,这会在膜表面产生一些暂时的疤痕。
更新日期:2022-09-19
中文翻译:

柠檬酸盐封端的超小金纳米粒子在支持的脂质双层界面上的原子分辨率行为
纳米材料具有改变生物和生物医学研究的潜力,其应用范围从药物输送和诊断到特定生物过程的靶向干扰。大多数现有研究旨在开发用于特定任务的纳米材料,例如增强生物细胞内化。然而,纳米材料与生物系统,特别是膜之间相互作用的基本方面仍然知之甚少。在这项研究中,我们对控制与模型磷脂膜接触的最常见合成纳米材料之一的相互作用和进化的分子机制提供了详细的见解。结合使用原子力显微镜 (AFM) 和分子动力学 (MD) 模拟,我们阐明了柠檬酸盐封端的 5 nm 金纳米粒子 (AuNP) 与纯流体 (DOPC) 和纯凝胶相 (DPPC) 磷脂的支持脂质双层 (SLB) 相互作用的精确机制。在液相 DOPC 膜上,随着 NPs 的柠檬酸盐帽被周围的脂质置换,AuNPs 吸附并逐渐内化。AuNPs 还与凝胶相 DPPC 膜相互作用,它们部分嵌入外叶,局部干扰脂质组织。在这两个系统中,AuNP 都会引起整个双层的整体扰动。AFM 显示颗粒的横向扩散比脂质分子的横向扩散小几个数量级,这会在膜表面产生一些暂时的疤痕。

































 京公网安备 11010802027423号
京公网安备 11010802027423号