Renewable and Sustainable Energy Reviews ( IF 16.3 ) Pub Date : 2022-09-15 , DOI: 10.1016/j.rser.2022.112918
Shangcong Sun , Qiuqiao Jiang , Dongyue Zhao , Tiantian Cao , Hao Sha , Chuankun Zhang , Haitao Song , Zhijian Da
|
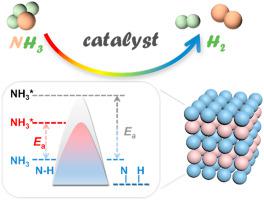
On-site ammonia decomposition has been considered as a potential candidate to alleviate the challenges of hydrogen storage and transportation by utilizing NH3 (with a high hydrogen content of 17.6 wt%) as hydrogen carrier, along with the flourish of renewable energy. Although the decomposition of NH3 into H2 is thermodynamically favorable at above 400 °C, the reaction kinetics remains sluggish due to the high activation energy for N–H bond cleavage and N2 desorption. This motivates the design and construction of functional catalysts with high-efficiency and low-cost. To date, a variety of metal-based catalysts have been investigated for NH3 decomposition, among which Ru often shows the highest activity due to its optimal metal–nitrogen binding energy. Efforts are being devoted to the further improvement of catalytic performance through tuning the morphology, electronic structure, defect/doping and metal–support interaction. Meanwhile, advanced techniques are employed to disclose the structure–performance relationship of catalyst. Herein, this review identifies the fundamental principles of catalytic NH3 decomposition, addresses the advances on current catalyst design, summarizes the strategies to enhancing efficiency, and provides recommendations for further material design. A comprehensive consideration of recent development of noble metal and transition metal catalysts are provided, as well as the promoters and supports. Moreover, the advantage of using bimetallic materials, which offers a synergy to modulate the electronic structure and improve the intrinsic activity, is emphasized. This review may serve as an informative work to inspire the future development of ammonia decomposition catalyst for practical application.
中文翻译:

氨作为氢载体:氨分解催化剂用于制氢的进展
随着可再生能源的蓬勃发展,现场氨分解被认为是利用NH 3(氢含量高达17.6 wt%)作为氢载体来缓解氢储存和运输挑战的潜在候选者。尽管 NH 3分解为 H 2在热力学上是有利的,温度高于 400 ℃,但由于 N-H 键断裂和 N 2解吸的高活化能,反应动力学仍然缓慢。这促进了高效、低成本的功能催化剂的设计和构建。迄今为止,已经研究了多种金属基催化剂用于 NH 3分解,其中Ru由于其最佳的金属-氮结合能而经常表现出最高的活性。正在努力通过调整形态、电子结构、缺陷/掺杂和金属-载体相互作用来进一步提高催化性能。同时,采用先进技术揭示催化剂的结构-性能关系。在此,本综述确定了催化 NH 3的基本原理分解,解决当前催化剂设计的进展,总结提高效率的策略,并为进一步的材料设计提供建议。综合考虑了贵金属和过渡金属催化剂的最新发展,以及促进剂和载体。此外,强调了使用双金属材料的优势,它提供了调节电子结构和提高内在活性的协同作用。该综述可作为一项信息性工作,以激发氨分解催化剂的实际应用的未来发展。































 京公网安备 11010802027423号
京公网安备 11010802027423号