Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-09-17 , DOI: 10.1016/j.cej.2022.139258 Baichao Zhang , Yunlong Xu , Brian Makuza , Fangjun Zhu , Haoji Wang , Ningyun Hong , Zhen Long , Wentao Deng , Guoqiang Zou , Hongshuai Hou , Xiaobo Ji
|
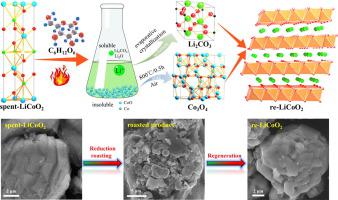
Due to the mounting pressure to mitigate environmental pollution and guarantee the sustainability of the battery metals, the recycling of spent lithium-ion batteries (LIBs) has become a crucial issue in recent years. Numerous studies have focused on utilizing high temperatures or strong acid/alkali to reduce the spent active cathode material to a low valence state, which exacerbates recycling and post-treatment costs. Herein, a low-temperature roasting process followed by a water leaching strategy is developed to recycle and regenerate LiCoO2 cathode material from spent LiCoO2 batteries. The spent LiCoO2 is roasted with glucose (C6H12O6) as a reagent to attain reduction of the cathode material into water-soluble Li salt (Li2O and Li2CO3) and water-insoluble (Co and CoO). The proposed method utilizes moderate roasting temperature and water as the only leaching reagent, which effectively reduces energy and chemical consumption. Li and Co recovery rates are 97 % and 99 %, respectively, under the optimal conditions of 1 h roasting at 550 °C followed by 30 min water leaching using a solid–liquid ratio of 50 g/L. The recovered Li-rich solution and Co-rich residue can be easily converted to Li2CO3 and Co3O4, respectively, which are the precursors for regenerating the LiCoO2 cathode material. The regenerated LiCoO2 exhibits superior cycling stability with 87 % capacity retention after 800 cycles at 4.4 V. This developed closed-loop recycling process has lower recycling costs, is eco-friendly and efficient, and thus could potentially promote the sustainable long-run development of the LIBs industry.
中文翻译:

从废旧锂离子电池中选择性提取锂并再生LiCoO2正极材料
由于减轻环境污染和保证电池金属可持续性的压力越来越大,废旧锂离子电池(LIB)的回收利用已成为近年来的一个关键问题。许多研究都集中在利用高温或强酸/碱将废活性正极材料还原为低价态,这会加剧回收和后处理成本。在此,开发了一种低温焙烧工艺,然后采用水浸出策略,以从废弃的 LiCoO 2电池中回收和再生 LiCoO 2正极材料。用过的 LiCoO 2用葡萄糖(C 6 H 12 O 6) 作为试剂将正极材料还原为水溶性锂盐(Li 2 O 和 Li 2 CO 3)和水不溶性(Co 和 CoO)。该方法利用适度的焙烧温度和水作为唯一的浸出剂,有效地降低了能源和化学品的消耗。在 550 °C 焙烧 1 小时,然后以 50 g/L 的固液比进行水浸 30 分钟的最佳条件下,Li 和 Co 的回收率分别为 97% 和 99%。回收的富锂溶液和富钴残渣可以很容易地分别转化为 Li 2 CO 3和 Co 3 O 4,它们是再生 LiCoO 2的前驱体阴极材料。再生的 LiCoO 2表现出优异的循环稳定性,在 4.4 V 下循环 800 次后容量保持率为 87%。这种开发的闭环回收工艺具有较低的回收成本、环保和高效,因此可能促进可持续的长期发展锂离子电池行业。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号