当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of the Molecular Structure on the Electrocatalytic Hydrogenation of Carbonyl Groups and H2 Evolution on Pd
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-16 , DOI: 10.1021/acscatal.2c03207 Laura C. Meyer 1 , Udishnu Sanyal 1 , Kelsey A. Stoerzinger 1, 2 , Katherine Koh 1 , John L. Fulton 1 , Donald M. Camaioni 1 , Oliver Y. Gutiérrez 1 , Johannes A. Lercher 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-09-16 , DOI: 10.1021/acscatal.2c03207 Laura C. Meyer 1 , Udishnu Sanyal 1 , Kelsey A. Stoerzinger 1, 2 , Katherine Koh 1 , John L. Fulton 1 , Donald M. Camaioni 1 , Oliver Y. Gutiérrez 1 , Johannes A. Lercher 1, 3
Affiliation
|
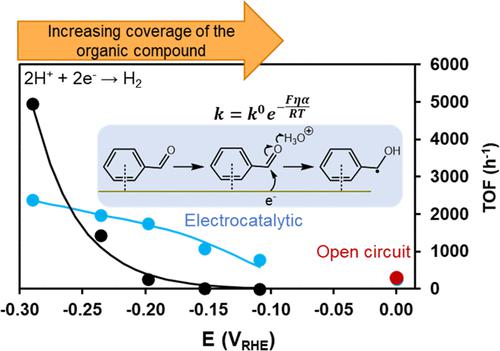
We investigated the electrocatalytic hydrogenation (ECH) of model aldehydes and ketones over carbon-supported Pd in the aqueous phase. We propose reaction mechanisms based on kinetic measurements and on spectroscopic and electrochemical characterization of the working catalyst. The reaction rates of ECH and of the H2 evolution reaction (HER) vary with the applied electric potential following trends that strongly depend on the organic substrate. The intrinsic rates of hydrogenation and H2 evolution are influenced, in opposing ways, by the sorption of the reacting organic substrate. Strong interactions, that is, higher standard free energies of adsorption of the organic compound, induce high hydrogenation rates. The fast hydrogenation kinetics produces a hydrogen-depleted environment that kinetically hinders the HER and the bulk phase transition of Pd to a H-rich bulk Pd hydride, which is triggered by the applied potential in the absence of reacting organic compounds. As a consequence of strong organic–metal interactions, hydrogenation dominates at low overpotential. However, the coverages of organic substrates on the metal surface decrease, and the rates of H2 evolution surpass those of hydrogenation with increasingly negative electric potential. We determined the range of electric potential favoring hydrogenation on Pd and quantitatively deconvoluted the effects of the sorption of the organic compound, and of the rates of proton-coupled electron transfers, on the kinetics of both ECH and HER. The results indicate that electrocatalysis offers hydrogenation pathways for polar molecules which are different and, in some cases, faster than those dominating in the absence of an external electric potential.
中文翻译:

分子结构对羰基电催化加氢和 Pd 析氢的影响
我们研究了水相中碳负载 Pd 上模型醛和酮的电催化氢化 (ECH)。我们提出了基于动力学测量以及工作催化剂的光谱和电化学表征的反应机制。ECH 和 H 2析出反应 (HER) 的反应速率随施加的电势而变化,遵循强烈依赖于有机底物的趋势。氢化和H 2的固有速率反应的有机底物的吸附以相反的方式影响演化。强相互作用,即有机化合物的更高标准吸附自由能,会导致高氢化率。快速加氢动力学产生了一个贫氢环境,该环境在动力学上阻碍了 HER 和 Pd 的本体相转变为富含 H 的本体 Pd 氢化物,这是在没有反应有机化合物的情况下由施加的电位触发的。由于强烈的有机-金属相互作用,氢化在低过电位下占主导地位。然而,金属表面的有机底物覆盖率降低,H 2进化超过了具有越来越负电势的氢化。我们确定了有利于 Pd 上加氢的电势范围,并定量地解卷积了有机化合物吸附和质子耦合电子转移速率对 ECH 和 HER 动力学的影响。结果表明,电催化为极性分子提供了不同的氢化途径,在某些情况下,它们比在没有外部电势的情况下占主导地位的分子更快。
更新日期:2022-09-16
中文翻译:

分子结构对羰基电催化加氢和 Pd 析氢的影响
我们研究了水相中碳负载 Pd 上模型醛和酮的电催化氢化 (ECH)。我们提出了基于动力学测量以及工作催化剂的光谱和电化学表征的反应机制。ECH 和 H 2析出反应 (HER) 的反应速率随施加的电势而变化,遵循强烈依赖于有机底物的趋势。氢化和H 2的固有速率反应的有机底物的吸附以相反的方式影响演化。强相互作用,即有机化合物的更高标准吸附自由能,会导致高氢化率。快速加氢动力学产生了一个贫氢环境,该环境在动力学上阻碍了 HER 和 Pd 的本体相转变为富含 H 的本体 Pd 氢化物,这是在没有反应有机化合物的情况下由施加的电位触发的。由于强烈的有机-金属相互作用,氢化在低过电位下占主导地位。然而,金属表面的有机底物覆盖率降低,H 2进化超过了具有越来越负电势的氢化。我们确定了有利于 Pd 上加氢的电势范围,并定量地解卷积了有机化合物吸附和质子耦合电子转移速率对 ECH 和 HER 动力学的影响。结果表明,电催化为极性分子提供了不同的氢化途径,在某些情况下,它们比在没有外部电势的情况下占主导地位的分子更快。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号