当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Emerging properties from mechanical tethering within a post-synthetically functionalised catenane scaffold
Chemical Science ( IF 7.6 ) Pub Date : 2022-09-16 , DOI: 10.1039/d2sc04101d Nadia Hoyas Pérez 1 , Peter S Sherin 1 , Victor Posligua 1 , Jake L Greenfield 1 , Matthew J Fuchter 1 , Kim E Jelfs 1 , Marina K Kuimova 1 , James E M Lewis 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-09-16 , DOI: 10.1039/d2sc04101d Nadia Hoyas Pérez 1 , Peter S Sherin 1 , Victor Posligua 1 , Jake L Greenfield 1 , Matthew J Fuchter 1 , Kim E Jelfs 1 , Marina K Kuimova 1 , James E M Lewis 1
Affiliation
|
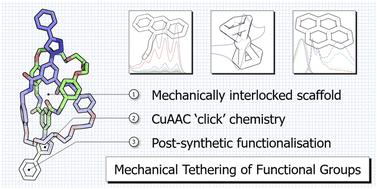
Maintaining close spatial proximity of functional moieties within molecular systems can result in fascinating emergent properties. Whilst much work has been done on covalent tethering of functional units for myriad applications, investigations into mechanically linked systems are relatively rare. Formation of the mechanical bond is usually the final step in the synthesis of interlocked molecules, placing limits on the throughput of functionalised architectures. Herein we present the synthesis of a bis-azide [2]catenane scaffold that can be post-synthetically modified using CuAAC ‘click’ chemistry. In this manner we have been able to access functionalised catenanes from a common precursor and study the properties of electrochemically active, emissive and photodimerisable units within the mechanically interlocked system in comparison to non-interlocked analogues. Our data demonstrates that the greater (co-)conformational flexibility that can be obtained with mechanically interlocked systems compared to traditional covalent tethers paves the way for developing new functional molecules with exciting properties.
中文翻译:

合成后功能化索烷支架内机械束缚的新兴特性
保持分子系统内功能部分的紧密空间接近性可以产生令人着迷的新兴特性。虽然在功能单元的共价束缚方面已经做了很多工作以用于无数的应用,但对机械连接系统的研究相对较少。机械键的形成通常是互锁分子合成的最后一步,这限制了功能化结构的产量。在此,我们介绍了双叠氮化物 [2] 链烯支架的合成,该支架可以使用 CuAAC“点击”化学进行合成后修饰。通过这种方式,我们能够从常见的前体中获得功能化的索烷,并研究机械联锁系统中电化学活性、发射和光二聚单元与非联锁类似物的性能。我们的数据表明,与传统的共价键相比,机械联锁系统可以获得更大的(共)构象灵活性,为开发具有令人兴奋的特性的新功能分子铺平了道路。
更新日期:2022-09-16
中文翻译:

合成后功能化索烷支架内机械束缚的新兴特性
保持分子系统内功能部分的紧密空间接近性可以产生令人着迷的新兴特性。虽然在功能单元的共价束缚方面已经做了很多工作以用于无数的应用,但对机械连接系统的研究相对较少。机械键的形成通常是互锁分子合成的最后一步,这限制了功能化结构的产量。在此,我们介绍了双叠氮化物 [2] 链烯支架的合成,该支架可以使用 CuAAC“点击”化学进行合成后修饰。通过这种方式,我们能够从常见的前体中获得功能化的索烷,并研究机械联锁系统中电化学活性、发射和光二聚单元与非联锁类似物的性能。我们的数据表明,与传统的共价键相比,机械联锁系统可以获得更大的(共)构象灵活性,为开发具有令人兴奋的特性的新功能分子铺平了道路。































 京公网安备 11010802027423号
京公网安备 11010802027423号