Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2022-09-16 , DOI: 10.1016/j.jcis.2022.09.068
Zhuo Liu 1 , Fei Guo 1 , Lei Cheng 2 , Xiangjie Bo 3 , Tingting Liu 4 , Mian Li 1
|
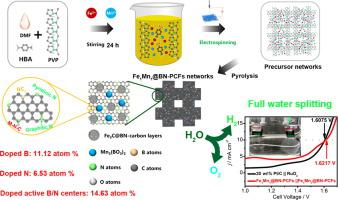
With high prices of precious metals (such as platinum, iridium, and ruthenium) and transition metals (such as cobalt and nickel), the design of high-efficiency and low-cost non-precious-metal-based catalysts using iron (Fe) and manganese (Mn) metals for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) are critical for commercial applications of water splitting devices. In the study, without using any template or surfactant, we successfully designed novel cross-linked manganese borate (Mn3(BO3)2) and iron carbide (Fe3C) embedded into boron (B) and nitrogen (N) co-doped three-dimensional (3D) hierarchically meso/macroporous carbon nanowires (denoted as FexMny@BN-PCFs). Electrochemical test results showed that the HER and OER catalytic activities of Fe1Mn1@BN-PCFs were close to those of 20 wt% Pt/C and RuO2. For full water splitting, (-) Fe1Mn1@BN-PCFs||Fe1Mn1@BN-PCF (+) cell achieved a current density of 10 mA cm−2 at a cell voltage of 1.622 V, which was 14.2 mV larger than that of (-) 20 wt% Pt/C||RuO2 (+) benchmark. The synergistic effect of 3D hierarchically meso/macroporous architectures, excellent charge transport capacity, and abundant active centers (cross-linked Mn3(BO3)2/Fe3C@BNC, BC3, pyridinic-N, M N
N C, and graphitic-N) enhanced the water splitting catalytic activity of Fe1Mn1@BN-PCFs. The (-) Fe1Mn1@BN-PCFs||Fe1Mn1@BN-PCF (+) cell exhibited excellent stability owing to the superior structural and chemical stabilities of 3D hierarchically porous Fe1Mn1@BN-PCFs.
C, and graphitic-N) enhanced the water splitting catalytic activity of Fe1Mn1@BN-PCFs. The (-) Fe1Mn1@BN-PCFs||Fe1Mn1@BN-PCF (+) cell exhibited excellent stability owing to the superior structural and chemical stabilities of 3D hierarchically porous Fe1Mn1@BN-PCFs.
中文翻译:

制备封装在氮和硼共掺杂碳纳米线中的硼酸锰/碳化铁作为加速碱性全水分解双功能电催化剂
由于贵金属(如铂、铱和钌)和过渡金属(如钴和镍)的价格较高,使用铁 (Fe) 和锰 (Mn) 金属进行析氢反应 (HER) 和析氧反应 (OER) 的高效、低成本非贵金属基催化剂的设计对于水分解装置的商业应用至关重要。在这项研究中,在不使用任何模板或表面活性剂的情况下,我们成功设计了新型交联硼酸锰 (Mn3(BO3)2) 和碳化铁 (Fe3C) 嵌入硼 (B) 和氮 (N) 共掺杂三维 (3D) 分层介孔/大孔碳纳米线 (表示为 FexMny@BN-PCFs)。电化学测试结果表明,Fe1Mn1@BN-PCFs 的 HER 和 OER 催化活性接近 20 wt% Pt/C 和 RuO2。对于完全分解水,(-) Fe1Mn1@BN-PCFs||Fe1Mn1@BN-PCF (+) 电池在 1.622 V 的电池电压下实现了 10 mA cm-2 的电流密度,比 (-) 20 wt% Pt/C 的电流密度高 14.2 mV||RuO2 (+) 基准测试。3D 层次介观/大孔结构、出色的电荷传输能力和丰富的活性中心 (交联 Mn3(BO3)2/Fe3C@BNC、BC3、吡啶-N、M ![]() N
N ![]() C 和石墨-N)的协同效应增强了 Fe1Mn1@BN-PCF 的水分解催化活性。 (-) Fe1Mn1@BN-PCFs||Fe1Mn1@BN-PCF (+) 电池由于 3D 分层多孔 Fe1Mn1@BN-PCF 具有出色的结构和化学稳定性,因此表现出优异的稳定性。
C 和石墨-N)的协同效应增强了 Fe1Mn1@BN-PCF 的水分解催化活性。 (-) Fe1Mn1@BN-PCFs||Fe1Mn1@BN-PCF (+) 电池由于 3D 分层多孔 Fe1Mn1@BN-PCF 具有出色的结构和化学稳定性,因此表现出优异的稳定性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号