International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2022-09-15 , DOI: 10.1016/j.ijhydene.2022.08.199
M. Abd Elkodous , Aziz Aatiqah , Go Kawamura , Wai Kian Tan , Atsunori Matsuda
|
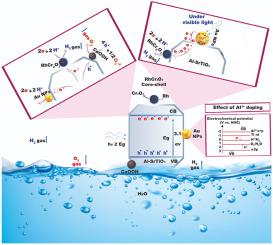
Developing new renewable, carbon-neutral fuels to diminish the amount of released CO2 in the atmosphere and to solve global challenges such as global warming and climate change is significant. Among them, hydrogen (H2) is attracting much attention due to its high energy density, ease of transportation, and multiple means of production. To meet the global demand of H2, photocatalytic water splitting is one of the most promising methods for large scale production. Herein, Al-doped SrTiO3 photocatalyst (Al–SrTiO3) was prepared by a molten flux method. Then, plasmonic metal nanoparticles (Au, Cu, Pt), and cocatalysts Rh/Cr2O3 and CoOOH were selectively deposited onto the reductive and oxidative active sites of Al–SrTiO3 using multi-step photodeposition-impregnation methods for water splitting and H2 production under UV-rays, UV–Vis. Light, and visible light (λ ≥ 400 nm). Our results showed that, compared with Pt and Cu loaded Al–SrTiO3 photocatalyst supported with Rh/Cr2O3 and CoOOH cocatalysts, Au-loaded samples showed the highest H2 production efficiency under both UV (920 μmol/h - EQE = 41% at 365 nm) and UV–Vis (100.5 μmol/h) rays. In addition, the amount of evolved H2 decreased by increasing the weight ratio of Au nanoparticles (NPs) due to the overlap between Au NPs and Rh/Cr2O3 cocatalyst. Although Au 0.3 wt%-loaded sample showed high activity under both UV and UV–Vis. Rays, it exhibited almost no efficiency under visible light because of the large bandgap of Al–SrTiO3 (3.1 eV) and the poor absorption in the visible region. Visible light absorption was then enhanced by increasing the loaded amount of Au NPs and by separating Au NPs and Rh/Cr2O3 cocatalyst responsible for H2 evolution by combining both photodeposition and impregnation methods. Under visible light, Rh/Cr2O3-loaded Al–SrTiO3 with 4 wt % Au NPs showed the highest H2 evolution efficiency (41 μmol/3 h). This was attributed to the efficient hot electron transfer from Au NPs to Al–SrTiO3 then to RhCr2O3, resulting in charge separation needed for efficient H2 generation.
中文翻译:

金属纳米粒子负载 Al-SrTiO3 负载 RhCr2O3 和 CoOOH 助催化剂用于整体水分解
开发新的可再生碳中和燃料以减少大气中释放的 CO 2量并解决全球变暖和气候变化等全球挑战具有重要意义。其中,氢(H 2)因其能量密度高、运输方便、生产方式多样等优点而备受关注。为满足全球对H 2 的需求,光催化水分解是最有前景的大规模生产方法之一。在此,Al掺杂的SrTiO 3光催化剂(Al-SrTiO 3)通过熔融焊剂法制备。然后,等离子金属纳米粒子(Au、Cu、Pt)和助催化剂 Rh/Cr 2 O 3采用多步光沉积-浸渍法,在紫外线、UV-Vis 下将CoOOH 和 CoOOH 选择性沉积到 Al-SrTiO 3的还原和氧化活性位点上,用于水分解和 H 2生成。光和可见光 (λ ≥ 400 nm)。我们的结果表明,与负载铂和铜的 Al-SrTiO 3光催化剂负载的 Rh/Cr 2 O 3和 CoOOH 助催化剂相比,负载 Au 的样品在两种紫外光下均表现出最高的 H 2生产效率(920 μmol/h - EQE = 41% at 365 nm) 和 UV-Vis (100.5 μmol/h) 射线。此外,析出的H 2由于 Au NPs 和 Rh/Cr 2 O 3助催化剂之间的重叠,通过增加 Au 纳米颗粒 (NPs) 的重量比而降低。尽管负载 Au 0.3 wt% 的样品在 UV 和 UV-Vis 下均显示出高活性。射线,它在可见光下几乎没有表现出效率,因为 Al-SrTiO 3的带隙大(3.1 eV)和可见光区域的吸收差。然后通过增加 Au NPs 的负载量和通过结合光沉积和浸渍方法分离 Au NPs 和负责 H 2释放的 Rh/Cr 2 O 3助催化剂来增强可见光吸收。可见光下,负载Rh/Cr 2 O 3的Al-SrTiO具有 4 wt% Au NPs 的图3显示出最高的 H 2释放效率(41 μmol/3 h)。这归因于从Au NPs 到Al-SrTiO 3再到RhCr 2 O 3的有效热电子转移,导致有效H 2生成所需的电荷分离。

































 京公网安备 11010802027423号
京公网安备 11010802027423号