Molecular Cell ( IF 14.5 ) Pub Date : 2022-09-15 , DOI: 10.1016/j.molcel.2022.08.018
Sihan Li 1 , Ken Ikeuchi 2 , Misaki Kato 1 , Robert Buschauer 3 , Takato Sugiyama 1 , Shungo Adachi 4 , Hideo Kusano 4 , Tohru Natsume 4 , Otto Berninghausen 3 , Yoshitaka Matsuo 5 , Thomas Becker 3 , Roland Beckmann 3 , Toshifumi Inada 5
|
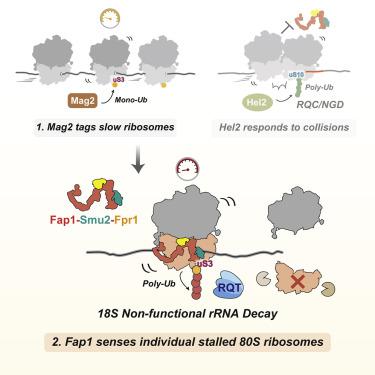
Cells can respond to stalled ribosomes by sensing ribosome collisions and employing quality control pathways. How ribosome stalling is resolved without collisions, however, has remained elusive. Here, focusing on noncolliding stalling exhibited by decoding-defective ribosomes, we identified Fap1 as a stalling sensor triggering 18S nonfunctional rRNA decay via polyubiquitination of uS3. Ribosome profiling revealed an enrichment of Fap1 at the translation initiation site but also an association with elongating individual ribosomes. Cryo-EM structures of Fap1-bound ribosomes elucidated Fap1 probing the mRNA simultaneously at both the entry and exit channels suggesting an mRNA stasis sensing activity, and Fap1 sterically hinders the formation of canonical collided di-ribosomes. Our findings indicate that individual stalled ribosomes are the potential signal for ribosome dysfunction, leading to accelerated turnover of the ribosome itself.
中文翻译:

Fap1 对单个停滞的 80S 核糖体进行非功能性 rRNA 转换的传感
细胞可以通过感知核糖体碰撞和采用质量控制途径来响应停滞的核糖体。然而,如何在不发生碰撞的情况下解决核糖体停滞仍然难以捉摸。在这里,专注于解码缺陷核糖体表现出的非碰撞停滞,我们将 Fap1 确定为一种停滞传感器,通过 uS3 的多泛素化触发 18S 非功能性 rRNA 衰变。核糖体分析揭示了 Fap1 在翻译起始位点的富集,但也与单个核糖体的延长有关。Fap1 结合核糖体的冷冻电镜结构阐明了 Fap1 在入口和出口通道同时探测 mRNA,表明 mRNA 停滞传感活性,并且 Fap1 在空间上阻碍了典型碰撞二核糖体的形成。




































 京公网安备 11010802027423号
京公网安备 11010802027423号