Cell ( IF 45.5 ) Pub Date : 2022-09-15 , DOI: 10.1016/j.cell.2022.08.008 Fu-Yang Lin 1 , Jing Li 1 , Yonghua Xie 2 , Jianghai Zhu 1 , Thi Thu Huong Nguyen 3 , Yonghui Zhang 2 , Jieqing Zhu 4 , Timothy A Springer 1
|
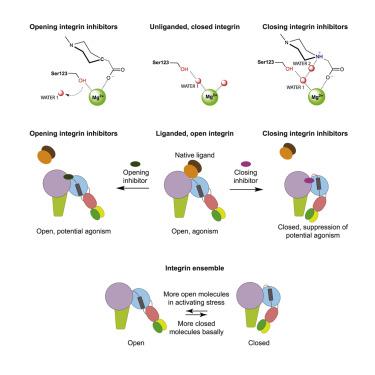
Integrins are validated drug targets with six approved therapeutics. However, small-molecule inhibitors to three integrins failed in late-stage clinical trials for chronic indications. Such unfavorable outcomes may in part be caused by partial agonism, i.e., the stabilization of the high-affinity, extended-open integrin conformation. Here, we show that the failed, small-molecule inhibitors of integrins αIIbβ3 and α4β1 stabilize the high-affinity conformation. Furthermore, we discovered a simple chemical feature present in multiple αIIbβ3 antagonists that stabilizes integrins in their bent-closed conformation. Closing inhibitors contain a polar nitrogen atom that stabilizes, via hydrogen bonds, a water molecule that intervenes between a serine residue and the metal in the metal-ion-dependent adhesion site (MIDAS). Expulsion of this water is a requisite for transition to the open conformation. This change in metal coordination is general to integrins, suggesting broad applicability of the drug-design principle to the integrin family, as validated with a distantly related integrin, α4β1.
中文翻译:

创建闭合稳定整合素抑制剂的一般化学原理
整合素是经过六种批准的治疗方法验证的药物靶点。然而,三种整合素的小分子抑制剂在慢性适应症的后期临床试验中失败了。这种不利的结果可能部分是由部分激动引起的,即高亲和力、扩展开放的整联蛋白构象的稳定。在这里,我们证明失败的整合素 αIIbβ3 和 α4β1 小分子抑制剂稳定了高亲和力构象。此外,我们发现多种 αIIbβ3 拮抗剂中存在一个简单的化学特征,可以稳定整合素的弯曲闭合构象。闭合抑制剂含有一个极性氮原子,通过氢键稳定介入丝氨酸残基和金属离子依赖性粘附位点(MIDAS)中的金属之间的水分子。排出这些水是转变为开放构象的必要条件。这种金属配位的变化对于整联蛋白来说是普遍存在的,这表明药物设计原理对整联蛋白家族具有广泛的适用性,这一点已通过远缘相关的整联蛋白α4β1进行了验证。































 京公网安备 11010802027423号
京公网安备 11010802027423号