当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A peptide-crosslinking approach identifies HSPA8 and PFKL as selective interactors of an actin-derived peptide containing reduced and oxidized methionine
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2022-09-15 , DOI: 10.1039/d2cb00183g
Aaron Maurais 1 , Eranthie Weerapana 1
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2022-09-15 , DOI: 10.1039/d2cb00183g
Aaron Maurais 1 , Eranthie Weerapana 1
Affiliation
|
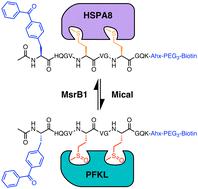
The oxidation of methionine to methionine sulfoxide occurs under conditions of cellular oxidative stress, and modulates the function of a diverse array of proteins. Enzymatic systems that install and reverse the methionine sulfoxide modifications have been characterized, however, little is known about potential readers of this oxidative modification. Here, we apply a peptide-crosslinking approach to identify proteins that are able to differentially interact with reduced and oxidized methionine-containing peptides. Specifically, we generated a photo-crosslinking peptide derived from actin, which contains two sites of methionine oxidation, M44 and M47. Our proteomic studies identified heat shock proteins, including HSPA8, as selective for the reduced methionine-containing peptide, whereas the phosphofructokinase isoform, PFKL, preferentially interacts with the oxidized form. We then demonstrate that the favored interaction of PFKL with oxidized methionine is also observed in the full-length actin protein, suggesting a role of methionine oxidation in regulating the actin-PFKL interaction in cells. Our studies demonstrate the potential to identify proteins that can differentiate between reduced and oxidized methionine and thereby mediate downstream protein functions under conditions of oxidative stress. Furthermore, given that numerous sites of methionine oxidation have now been identified, these studies set the stage to identify putative readers of methionine oxidation on other protein targets.
中文翻译:

肽交联方法将 HSPA8 和 PFKL 鉴定为含有还原和氧化蛋氨酸的肌动蛋白衍生肽的选择性相互作用子
蛋氨酸氧化成蛋氨酸亚砜是在细胞氧化应激条件下发生的,并调节多种蛋白质的功能。安装和逆转甲硫氨酸亚砜修饰的酶系统已被表征,然而,人们对这种氧化修饰的潜在读者知之甚少。在这里,我们应用肽交联方法来鉴定能够与含还原和氧化蛋氨酸的肽有差异地相互作用的蛋白质。具体来说,我们生成了一种源自肌动蛋白的光交联肽,其中包含两个蛋氨酸氧化位点:M44 和 M47。我们的蛋白质组学研究发现,热休克蛋白(包括 HSPA8)对含还原甲硫氨酸的肽具有选择性,而磷酸果糖激酶异构体 PFKL 优先与氧化形式相互作用。然后我们证明,在全长肌动蛋白中也观察到 PFKL 与氧化蛋氨酸的有利相互作用,表明蛋氨酸氧化在调节细胞中肌动蛋白-PFKL 相互作用中的作用。我们的研究表明,有可能识别出能够区分还原蛋氨酸和氧化蛋氨酸的蛋白质,从而在氧化应激条件下介导下游蛋白质功能。此外,鉴于现已确定了许多蛋氨酸氧化位点,这些研究为确定其他蛋白质靶标上蛋氨酸氧化的假定读者奠定了基础。
更新日期:2022-09-15
中文翻译:

肽交联方法将 HSPA8 和 PFKL 鉴定为含有还原和氧化蛋氨酸的肌动蛋白衍生肽的选择性相互作用子
蛋氨酸氧化成蛋氨酸亚砜是在细胞氧化应激条件下发生的,并调节多种蛋白质的功能。安装和逆转甲硫氨酸亚砜修饰的酶系统已被表征,然而,人们对这种氧化修饰的潜在读者知之甚少。在这里,我们应用肽交联方法来鉴定能够与含还原和氧化蛋氨酸的肽有差异地相互作用的蛋白质。具体来说,我们生成了一种源自肌动蛋白的光交联肽,其中包含两个蛋氨酸氧化位点:M44 和 M47。我们的蛋白质组学研究发现,热休克蛋白(包括 HSPA8)对含还原甲硫氨酸的肽具有选择性,而磷酸果糖激酶异构体 PFKL 优先与氧化形式相互作用。然后我们证明,在全长肌动蛋白中也观察到 PFKL 与氧化蛋氨酸的有利相互作用,表明蛋氨酸氧化在调节细胞中肌动蛋白-PFKL 相互作用中的作用。我们的研究表明,有可能识别出能够区分还原蛋氨酸和氧化蛋氨酸的蛋白质,从而在氧化应激条件下介导下游蛋白质功能。此外,鉴于现已确定了许多蛋氨酸氧化位点,这些研究为确定其他蛋白质靶标上蛋氨酸氧化的假定读者奠定了基础。

































 京公网安备 11010802027423号
京公网安备 11010802027423号