当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical Dearomative Cycloadditions of Quinolines and Alkenes: Scope and Mechanism Studies
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-15 , DOI: 10.1021/jacs.2c07726
Renyu Guo 1 , Souvik Adak 1 , Peter Bellotti 2 , Xinfeng Gao 1 , W Walker Smith 1 , Sam Ngan Le 3 , Jiajia Ma 2 , K N Houk 4 , Frank Glorius 2 , Shuming Chen 3 , M Kevin Brown 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-15 , DOI: 10.1021/jacs.2c07726
Renyu Guo 1 , Souvik Adak 1 , Peter Bellotti 2 , Xinfeng Gao 1 , W Walker Smith 1 , Sam Ngan Le 3 , Jiajia Ma 2 , K N Houk 4 , Frank Glorius 2 , Shuming Chen 3 , M Kevin Brown 1
Affiliation
|
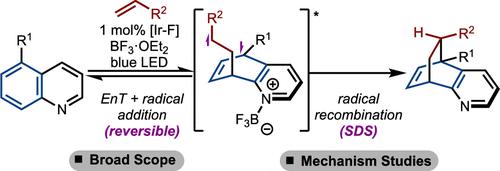
Photochemical dearomative cycloaddition has emerged as a useful strategy to rapidly generate molecular complexity. Within this context, stereo- and regiocontrolled intermolecular para-cycloadditions are rare. Herein, a method to achieve photochemical cycloaddition of quinolines and alkenes is shown. Emphasis is placed on generating sterically congested products and reaction of highly substituted alkenes and allenes. In addition, the mechanistic details of the process are studied, which revealed a reversible radical addition and a selectivity-determining radical recombination. The regio- and stereochemical outcome of the reaction is also rationalized.
中文翻译:

喹啉和烯烃的光化学脱芳环加成:范围和机制研究
光化学脱芳环加成已成为快速产生分子复杂性的有用策略。在这种情况下,立体和区域控制的分子间对环加成很少见。本文展示了一种实现喹啉和烯烃光化学环加成的方法。重点放在生成空间拥挤的产物以及高度取代的烯烃和丙二烯的反应上。此外,还研究了该过程的机制细节,揭示了可逆的自由基加成和选择性决定的自由基重组。反应的区域和立体化学结果也合理化。
更新日期:2022-09-15
中文翻译:

喹啉和烯烃的光化学脱芳环加成:范围和机制研究
光化学脱芳环加成已成为快速产生分子复杂性的有用策略。在这种情况下,立体和区域控制的分子间对环加成很少见。本文展示了一种实现喹啉和烯烃光化学环加成的方法。重点放在生成空间拥挤的产物以及高度取代的烯烃和丙二烯的反应上。此外,还研究了该过程的机制细节,揭示了可逆的自由基加成和选择性决定的自由基重组。反应的区域和立体化学结果也合理化。































 京公网安备 11010802027423号
京公网安备 11010802027423号