Fuel ( IF 6.7 ) Pub Date : 2022-09-14 , DOI: 10.1016/j.fuel.2022.125944
Tianhao Shen , Fengxia Zhang , Shiliang Yang , Yaohuan Wang , Huili Liu , Hua Wang , Jianhang Hu
|
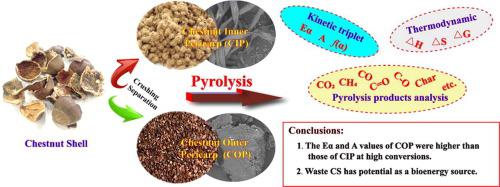
Chestnut shell (CS) is a potential renewable energy source as a typical agricultural waste. CS obtained two kinds of samples of chestnut outer pericarp (COP) and chestnut inner pericarp (CIP) with widely different physicochemical properties by crushing and separation. The kinetic triplet (apparent activation energy (Eα), pre-exponential factor (A), reaction mechanism function (f(α)), thermodynamic and products of COP and CIP are investigated by TGA, FTIR and SEM. Moreover, the Eα and the A are estimated using three isoconversional methods. The value of Eα for COP ranges from 155.63 to 392.95 kJ/mol and the CIP ranges from 185.24 to 337.03 kJ/mol. The Eα and A of CIP are found to be much smaller than COP at high conversion α, indicating that CIP is more susceptible to pyrolysis than COP. The 18 commonly theoretical models can only confirm that the reaction mechanism of COP at low α satisfies the A3, F1, R3 and D3 models, and the CIP satisfies the F1.5, R3, Pn and D1 models. Therefore, the empirical model (Šesták-Berggren) is used to determine f(α) for the whole pyrolysis process of COP and CIP. Through thermodynamic calculations, both CIP and COP were found to have a high potential to generate products as bioenergy through pyrolysis. The analysis of pyrolysis products shows that CIP can release more gaseous products (e.g., CH4, CO and CO2) during pyrolysis due to different physicochemical properties. COP-char and CIP-char exhibited two completely different microscopic morphologies of dense masses and loose bands. The results obtained can contribute to understand the thermal behavior of the CS pyrolysis process and the properties of the pyrolysis products, and give a reference for the high-value utilization of CS.
中文翻译:

板栗加工废料(板栗壳)热解过程综合研究:动力学三重态、热力学、放出气体原位监测和分析生物炭
板栗壳(CS)是一种潜在的可再生能源,是一种典型的农业废弃物。CS通过粉碎分离得到理化性质差异较大的板栗外皮(COP)和板栗内皮(CIP)两种样品。通过TGA、FTIR和SEM研究了COP和CIP的动力学三重态(表观活化能(Eα)、指前因子(A)、反应机理函数(f(α))、热力学和产物。此外,E α和 A 采用三种等转化方法估算,COP 的 E α值范围为 155.63 至 392.95 kJ/mol,CIP 范围为 185.24 至 337.03 kJ/mol 。发现 CIP 的 A 和 A 在高转化率 α 下远小于 COP,表明 CIP 比 COP 更容易热解。18个常用理论模型只能证实COP在低α的反应机理满足A3、F1、R3和D3模型,CIP满足F1.5、R3、Pn和D1模型。因此,经验模型 (Šesták-Berggren) 用于确定COP 和 CIP 整个热解过程的f (α)。通过热力学计算,发现 CIP 和 COP 都具有通过热解产生生物能源产品的高潜力。对热解产物的分析表明,CIP 可以释放更多的气态产物(如 CH 4、CO 和 CO 2) 在热解过程中由于不同的物理化学性质。COP-char和CIP-char表现出两种完全不同的致密团块和松散条带的微观形态。所得结果有助于了解CS热解过程的热行为和热解产物的性质,为CS的高价值利用提供参考。


































 京公网安备 11010802027423号
京公网安备 11010802027423号