当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic Characterization of a Diferric Mycobacterial Hemerythrin-Like Protein with Unprecedented Reactivity toward Nitric Oxide
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-13 , DOI: 10.1021/jacs.2c07113 Therese Albert 1 , Pierre Moënne-Loccoz 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-09-13 , DOI: 10.1021/jacs.2c07113 Therese Albert 1 , Pierre Moënne-Loccoz 1
Affiliation
|
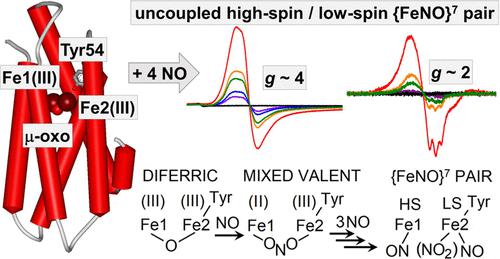
Hemerythrin-like proteins (HLPs) are broadly distributed across taxonomic groups and appear to play highly diverse functional roles in prokaryotes. Mycobacterial HLPs contribute to the survival of these pathogenic bacteria in mammalian macrophages, but their modes of action remain unclear. A recent crystallographic characterization of Mycobacterium kansasii HLP (Mka-HLP) revealed the unexpected presence of a tyrosine sidechain (Tyr54) near the coordination sphere of one of the two iron centers. Here, we show that Tyr54 is a true ligand to the Fe2(III) ion which, in conjunction with the presence of a μ-oxo group bridging the two iron(III), brings unique reactivity toward nitric oxide (NO). Monitoring the titration of Mka-HLP with NO by Fourier-transform infrared and electron paramagnetic resonance spectroscopies shows that both diferric and diferrous forms of Mka-HLP accumulate an uncoupled high-spin and low-spin {FeNO}7 pair. We assign the reactivity of the diferric protein to an initial radical reaction between NO and the μ-oxo bridge to form nitrite and a mixed-valent diiron center that can react further with NO. Amperometric measurements of NO consumption by Mka-HLP confirm that this reactivity can proceed at low micromolar concentrations of NO, before additional NO consumption, supporting a NO scavenging role for mycobacterial HLPs.
中文翻译:

对一氧化氮具有前所未有的反应性的二铁分枝杆菌类血红素蛋白的光谱表征
类血红素蛋白 (HLP) 广泛分布于分类群中,并且似乎在原核生物中发挥着高度多样化的功能作用。分枝杆菌 HLP 有助于这些病原菌在哺乳动物巨噬细胞中的存活,但它们的作用方式仍不清楚。最近对堪萨斯分枝杆菌HLP (Mka-HLP) 的晶体学表征揭示了在两个铁中心之一的配位球附近意外存在酪氨酸侧链 (Tyr54)。在这里,我们表明 Tyr54 是 Fe2(III) 离子的真正配体,与连接两个铁 (III) 的 μ-氧代基团的存在相结合,为一氧化氮 (NO) 带来了独特的反应性。监测Mka的滴定-HLP with NO 通过傅里叶变换红外和电子顺磁共振光谱显示,Mka -HLP 的二铁和二铁形式都积累了未耦合的高自旋和低自旋 {FeNO} 7对。我们将二铁蛋白的反应性归因于 NO 和 μ-氧代桥之间的初始自由基反应,以形成亚硝酸盐和可以与 NO 进一步反应的混合价二铁中心。通过Mka -HLP对 NO 消耗的电流测量证实,这种反应可以在低微摩尔浓度的 NO 下进行,在额外的 NO 消耗之前,支持分枝杆菌 HLP 的 NO 清除作用。
更新日期:2022-09-13
中文翻译:

对一氧化氮具有前所未有的反应性的二铁分枝杆菌类血红素蛋白的光谱表征
类血红素蛋白 (HLP) 广泛分布于分类群中,并且似乎在原核生物中发挥着高度多样化的功能作用。分枝杆菌 HLP 有助于这些病原菌在哺乳动物巨噬细胞中的存活,但它们的作用方式仍不清楚。最近对堪萨斯分枝杆菌HLP (Mka-HLP) 的晶体学表征揭示了在两个铁中心之一的配位球附近意外存在酪氨酸侧链 (Tyr54)。在这里,我们表明 Tyr54 是 Fe2(III) 离子的真正配体,与连接两个铁 (III) 的 μ-氧代基团的存在相结合,为一氧化氮 (NO) 带来了独特的反应性。监测Mka的滴定-HLP with NO 通过傅里叶变换红外和电子顺磁共振光谱显示,Mka -HLP 的二铁和二铁形式都积累了未耦合的高自旋和低自旋 {FeNO} 7对。我们将二铁蛋白的反应性归因于 NO 和 μ-氧代桥之间的初始自由基反应,以形成亚硝酸盐和可以与 NO 进一步反应的混合价二铁中心。通过Mka -HLP对 NO 消耗的电流测量证实,这种反应可以在低微摩尔浓度的 NO 下进行,在额外的 NO 消耗之前,支持分枝杆菌 HLP 的 NO 清除作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号