Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2022-09-13 , DOI: 10.1016/j.jpba.2022.115041 Cindy Kristina Enggi 1 , Fitrah Mahardika 1 , Delly Mayari Devara 1 , Mesakh Diki Saputra 1 , Nurfadilla Wafiah 1 , Muhammad Raihan 1 , Andi Dian Permana 1
|
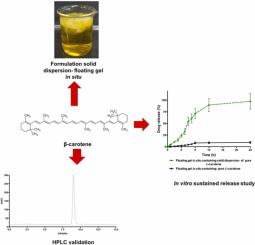
Despite the health benefits of β-carotene, its activity has been hampered by poor aqueous solubility and low oral bioavailability. Therefore, it is crucial to develop a new approach to overcome these problems. In this study, we developed a dry powder supplement comprising a combination approach of solid dispersion and floating gel in situ of β-carotene to enhance the solubility and achieve sustained release behavior. Here, we validated an HPLC method to quantify β-carotene as per the guidelines from ICH. The analytical method was validated in methanol and Fasted-State Simulated Gastric Fluid (FaSSGF) to determine β-carotene in recovery and in vitro release studies, respectively. A simple HPLC method using Xselect CSH™ C18 column (Waters, 3.0 × 150 mm) with the particle size of 3.5 µm was validated with 100% acetonitrile as the mobile phase. The calibration curves were found to be linear with LLOQ values < 3 ng/mL. Importantly, the method was accurate and precise without a carry over effect and successfully applied to determine the β-carotene concentration in the content analysis of the compound and in vitro drug release from floating gel in situ laden with solid dispersion formulations. The sensitivity of the method obtained here offers a wide potential use in various applications in drug delivery systems.
中文翻译:

HPLC-UV 方法验证在开发含有原位固体分散体浮动凝胶的缓释补充剂制剂中定量 β-胡萝卜素
尽管β-胡萝卜素对健康有益,但其活性受到水溶性差和口服生物利用度低的阻碍。因此,开发一种新的方法来克服这些问题至关重要。在这项研究中,我们开发了一种干粉补充剂,包括固体分散体和β-胡萝卜素原位浮动凝胶的组合方法,以提高溶解度并实现缓释行为。在这里,我们根据 ICH 的指南验证了一种 HPLC 方法来量化 β-胡萝卜素。该分析方法在甲醇和禁食状态模拟胃液 (FaSSGF) 中进行了验证,以确定回收和体外的 β-胡萝卜素分别发布研究。使用粒径为 3.5 µm 的 Xselect CSH™ C18 色谱柱(Waters,3.0 × 150 mm)的简单 HPLC 方法以 100% 乙腈为流动相进行了验证。发现校准曲线呈线性,LLOQ 值 < 3 ng/mL。重要的是,该方法准确无误,无残留效应,并成功应用于确定化合物含量分析中的 β-胡萝卜素浓度,以及载有固体分散体制剂的原位漂浮凝胶的体外药物释放。此处获得的方法的灵敏度为药物输送系统的各种应用提供了广泛的潜在用途。































 京公网安备 11010802027423号
京公网安备 11010802027423号