Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2022-09-09 , DOI: 10.1016/j.bmc.2022.117002 Takahito Tomori 1 , Koya Uekusa 1 , Aya Koyama 1 , Takayuki Kanagawa 1 , Yoshiaki Masaki 2 , Kohji Seio 1
|
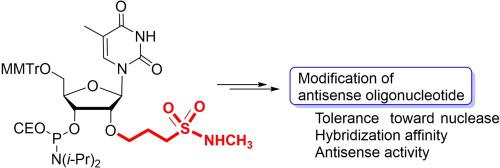
A synthetic scheme was developed to derive a modified ribothymidine bearing a 3-(N-methylsulfamoyl)propyl group on 2′-oxygen (TMSP). For synthesis initiation, a nucleophilic attack of 1,2-ethanediol on 5′-protected 2,2′-anhydro-ribothymidine was performed to selectively modify the 2′-position. After protection of the 3′-hydroxy group, the hydroxyethyl group was oxidized to the aldehyde, which was coupled with isobutyl (diethoxyphosphinyl)methanesulfonate through the Horner–Wadsworth–Emmons reaction to yield the sulfonate intermediate. The intermediate was further converted to the desired TMSP. Using the phosphoramidite units derived from nucleosides, we synthesized oligonucleotides incorporating TMSP. Oligonucleotides modified with TMSP were found to have duplex stability, resistance toward 3′-exonuclease digestion, and antisense activity comparable to that of the oligonucleotide modified with a previously reported 2′-O-methylcarbamoylethyl group. Based on these results and the generality of the synthetic scheme, 2′-O-sulfamoylalkyl modification is expected to be used for the modulation of the properties of oligonucleotides by changing the substituents on the nitrogen, enabling the oligonucleotides to possess suitable properties for antisense oligonucleotides.
中文翻译:

2'-O-[3-(N-methylsulfamoyl)propan-1-yl]ribothymidine 作为反义寡核苷酸潜在适用的 2'-修饰核苷的合成
开发了一种合成方案以衍生在 2'-氧 (T MSP ) 上带有 3-( N-甲基氨磺酰基)丙基的改性核胸苷。对于合成起始,1,2-乙二醇对 5'-保护的 2,2'-脱水-核胸苷进行亲核攻击以选择性地修饰 2'-位置。保护 3'-羟基后,羟乙基被氧化成醛,醛与异丁基(二乙氧基膦基)甲磺酸酯通过 Horner-Wadsworth-Emmons 反应生成磺酸酯中间体。中间体进一步转化为所需的 T MSP。使用源自核苷的亚磷酰胺单元,我们合成了包含 T MSP的寡核苷酸。用 T 修饰的寡核苷酸发现MSP具有双链体稳定性、对 3'-外切核酸酶消化的抗性以及与用先前报道的 2'- O-甲基氨基甲酰基乙基修饰的寡核苷酸相当的反义活性。基于这些结果和合成方案的一般性,2' - O-氨磺酰基烷基修饰有望通过改变氮上的取代基来调节寡核苷酸的性质,使寡核苷酸具有适合反义寡核苷酸的性质.














































 京公网安备 11010802027423号
京公网安备 11010802027423号